| Pages:
1
2 |
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
A small point, but no mention of Arsenic trioxide, which may be significant if one is working with Arsenic compounds. To quote:
"Arsenic trioxide is an amphoteric oxide, and its aqueous solutions are weakly acidic. Thus, it dissolves readily in alkaline solutions to give
arsenites. It is less soluble in acids, although it will dissolve in hydrochloric acid."
Arsenic trioxide - Wikipedia, the free encyclopedia
Wikipedia › wiki › Arsenic_trioxide
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@AJ:
The list wasn't intended to be definitive (I've added common Cr and Sb now).
Besides elements that from water-soluble hydroxy-anions, there are also some d-block elements that form oxo-anions like manganates, permanganates,
titanates, tantalates, ferrates and zirconates. Also: their fluoro-substituted analogues (Mn, Ti, Ta and Xr).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Acids in non-aqueous media and super acids:
We started this thread by stating:
| Quote: | An acid can be most simply defined as a proton donor, a base as a proton receptor. For a generic acid HA and base B,
the proton (H<sup>+</sup> exchange can be summarised as: exchange can be summarised as:
|
$$\mathrm{HA} + \mathrm{B} \leftrightarrow \mathrm{BH^+} + \mathrm{A^-}$$
For water:
$$2H_2O(l) \leftrightarrow H_3O^+(aq) + OH^-(aq)$$
Strong acids are acids that completely deprotonate:
$$HA + H_2O \to H_3O^+ + A^-$$
They do so because they are stronger acids than the oxonium ion.
This leads to the so-called levelling effect:
Acids that are stronger than oxonium completely deprotonate and cannot be distinguished from each other in water (on acid strength).
The well known strong mineral acids cannot be ranked in water:
$$HClO_4=HCl=H_2SO_4=HNO_3$$
But in a solvent like glacial acetic acid (anh. ethanoic acid) this is no longer true because of the auto-dissociation:
$$2HOAc \leftrightarrow H_2OAc^+ + OAc^-$$
The structure (with resonance) of protonated ethanoic acid is:
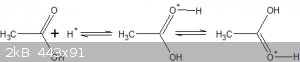
The protonated species of a non-aqueous solvent is referred to as the solvonium ion.
Now the mineral acids are weaker than H<sub>2</sub>OAc<sup>-</sup> and they are only partly deprotonated, in the order:
$$HClO_4>HCl>H_2SO_4>HNO_3$$
So in glacial acetic acid, the mineral acids behave like weak acids: only partly deprotonated and without levelling effect.
A frequently used application of this type of chemistry is non-aqueous acidometry of very weak bases. Take pyrazole e.g. With a pK<sub>B</sub> = 11.5, a 0.1 M solution in water would have an estimated pH of about 7.8, so it can’t be
titrated in water.
Below are the (potentiometric) titration curves of pyrazole and other weak bases in GAA with 0.1 M HClO<sub>4</sub> in GAA:

Note how, like in water, larger and steeper potential jumps are obtained for bases with smaller pK<sub>B</sub>.
GAA allows the titration of bases with a pK<sub>B</sub> up to about 12.
The titration of weak acids, in particular those insoluble in water (like higher fatty acids) in non-aqueous solvent is also practiced for analytical
purposes.
An example is the titration of higher fatty acids in methanol/benzene mixtures. The titrant solution is obtained by dissolving a known quantity of Na
metal in dry methanol in the absence of CO<sub>2</sub>, forming the strong base sodium methoxide. Dry benzene is then added to a
volumetric ratio of about 5 (benzene/MeOH) to finalise the titrant solution. A solution of Thymol blue in MeOH is used as indicator to titrate the
fatty acid (and other weak acids). Titration is performed under nitrogen to a blue equivalence point.
Super acids
The most interesting of the super acids are the non-HF based, non-corrosive carborane acids, which were introduced here.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
subtlety and pedantry:
Strictly speaking, basicity (ie, what pOH measures) is distinct from alkalinity (defined as a solution's acid-neutralizing power).
So-called reaction constants, including dissociation constants, are in fact variable, depending upon temperature and pressure. The effect is small enough to be disregarded in most situations, but the difference between a pKw of 14.0
at STP and 14.9 at 0 C might be relevant in a precision analytical setting, and the effect of pressure on reaction equilibria is an important
consideration in the chemistry of sea water.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks mayko. No contest.
|
|
|
| Pages:
1
2 |