| Pages:
1
2 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Acids and Bases: Brønsted–Lowry Theory (an interactive educational thread)
I’m starting this thread in ‘interactive seminar form’ for the benefit of beginners and burgeoning chemists who haven’t had the benefit of a
higher education in science or chemistry and want to develop a better understanding of what makes acids and bases tick.
Being a normal thread anyone is welcome (and it’s FREE!  ) to contribute,
correct me or ask questions. Of more knowledgeable contributors I ask only to take one thing into account: this thread is not about cutting edge
chemical theory, rather because of its stated intent shouldn’t really exceed A-level/1st year Uni levels of sophistication. If interest warrants it,
we can always build up. ) to contribute,
correct me or ask questions. Of more knowledgeable contributors I ask only to take one thing into account: this thread is not about cutting edge
chemical theory, rather because of its stated intent shouldn’t really exceed A-level/1st year Uni levels of sophistication. If interest warrants it,
we can always build up.
To improve searchability frequent thread navigation posts will be included.
<hr>
Upcoming instalments are:
1. Water as an acid and a base
2. Weak and strong acids and bases: Acid/base equilibrium constants
3. Essential pH calculations
[Edited on 20-1-2016 by blogfast25]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Excellent--this stuff confuses me. looking forward to the first installment.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Water as an acid and a base:
An acid can be most simply defined as a proton donor, a base as a proton receptor. For a generic acid HA and base B,
the proton (H<sup>+</sup> exchange can be summarised as: exchange can be summarised as:
HA+B↔BH++A−
Water, the most used solvent, is itself both an acid (albeit a very weak one) and a base because of the auto-dissociation reaction:
2H2O↔H3O++OH−
The formed cation is called the oxonium ion (in some texts: ‘hydronium ion’) and the anion the hydroxide ion.
As we’ll see later on, the auto-dissociation equilibrium leans far to the left and the concentrations of oxonium and hydroxide ions in pure, neutral
water are very, very small. But the fact that water can act as proton receptor or proton donor is the basis of Brønsted–Lowry theory.
When an acid dissolves in water we can now re-write our first reaction equation as follows:
HA+H2O↔H3O++A−
And when a base dissolves in water we can now re-write our first reaction equation as follows:
B+H2O↔BH++OH−
From here we can understand that in Brønsted–Lowry theory acidity/alkalinity is a concept that is relative to the
acidity/alkalinity of water! An acid is a proton donor that is stronger than water and a base is a proton receptor that is stronger than water.
<hr>
Notes:
1. Acid base reactions can also take place in other, protonatable solvents and are the subject of other theories. We’ll only concern ourselves with
non-aqueous acid base reactions at the end of this seminar.
2. Further reading for the curious: why water forms oxonium ions (scroll to below the fold).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Weak and strong acids and bases: Acid/base equilibrium constants
Firstly a quick elucidation of the concept of chemical equilibrium reaction. Take a simple example:
A+B↔C
In reality two reactions are taking place at once:
a. Forward reaction:
A+B→C
b. Reverse reaction:
A+B←C
Kinetically the reaction rates for both reactions can be found from (where indexed C indicate actual concentrations and k rate constants):
vforward=kfCACB
vreverse=krCC
When the equilibrium has been established the reaction these reaction rates have become constant and we can write:
krCCkfCACB=K′
And:
CCCACB=K
With K the equilibrium constant of the equilibrium reaction.
The expression for K is a mathematical condition imposed on the concentrations of reactants and reaction products which applies for a given pressure
and temperature.
We can distinguish two broad cases:
1. Right leaning equilibrium:
K>>1
Such an equilibrium is right leaning. In the limit:
K→∞
... the equilibrium reaction runs to the right to completion.
2. Left leaning equilibrium:
K<<1
Such an equilibrium is left leaning. In the limit:
K→0
... the reaction doesn’t proceed at all.
Let’s now apply this approach to the last two reactions of the previous post.
Acid de-protonation:
HA+H2O↔H3O++A−
Base protonation:
B+H2O↔BH++OH−
For ease of notation I will denote concentration of oxonium as:
CH
And the concentration of hydroxide as:
COH
For the acid de-protonation we get:
K=CHCACHACH2O
For dilute solutions:
CH2O≈55mol/L
So we can combine this with K to give:
KA=CHCACHA
Similarly for the protonation of a base:
KB=CBHCOHCB
A strong acid can now be defined as:
KA≫1
A weak one as:
KA≪1
A strong base can now be defined as:
KB≫1
A weak one as:
KB≪1
In the case of the well-known very strong mineral acids like sulphuric acid, nitric acid, hydrochloric acid (and some others) it’s safe to simplify
to:
KA≈∞
The de-protonation reaction of such acid runs to completion:
HA+H2O→H3O++A−
The solution of a very strong acid basically contains no ‘free acid’.
And what about water itself? For the auto-dissociation of water we can write the following equation:
KW=CHCOH=10−14mol2/L2
In pure, neutral water:
CH=COH=10−7mol/L
<hr>
Notes:
1. Critics may want to point out that strictly speaking chemical activities, rather than concentrations need to be used here and they would be
correct. We’re applying the theory here for dilute solutions where activity can be approximated by concentration.
2. The acid/base equilibrium constants for some common acids and bases can be found here, expressed as pK values (we’ll get to the concept of pK and pH shortly).
[Edited on 20-1-2016 by blogfast25]
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Wonderful use of formatting there Blogfast. A Slightly off-topic question:
You mentioned Chemical activities should be used, but I'm not quite sure what they are. The wiki page mostly just says that it's an "effective
concentration", but I don't really understand that. What exactly is a chemical activity, and why is it that they are measured using vapor pressures?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Oscilllator  | Wonderful use of formatting there Blogfast. A Slightly off-topic question:
You mentioned Chemical activities should be used, but I'm not quite sure what they are. The wiki page mostly just says that it's an "effective
concentration", but I don't really understand that. What exactly is a chemical activity, and why is it that they are measured using vapor pressures?
|
Thanks. The formatting is the hardest part.
Behind all this lies some nifty thermodynamics (not shown here), in particular the relation between equilibrium constants and Gibbs Free Energy via
Nernst and this requires the use of chemical activities rather than measurable concentrations.
This arises from the fact that these theories assume reacting particles to be totally isolated but at higher concentrations they tend to cluster
together, see e.g. Debeye Huckel Theory.
At infinite dilution activity and concentration are the same but at practical concentrations a correction needs to be applied. I'm assuming here that
in fairly dilute solutions the correction factor gamma (the 'activity coefficient'):
a=γC
... equals 1.
See also ionic strength.
[Edited on 20-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Essential pH calculations:
The concept of pH probably doesn’t require much introduction but for the sake of completeness:
pH=−logCH
Similarly we can define a pOH:
pOH=−logCOH
Using the auto-dissociation of water equation:
KW=CHCOH=10−14mol2/L2
By taking the - log of both sides we get:
pH+pOH=pKW=14
So at STP conditions the sum of pH and pOH is always 14!
The p-function can also be extended to acid base equilibrium constants:
pKA,B=−logKA,B
Note that the larger the value of pK<sub>A,B</sub> is the weaker the relevant acid or base is.
Oxonium/hydroxide concentrations for dilute solutions:
Let C be the nominal concentration of the acid or base, in mol/L.
I. Solutions of strong acids and bases (dilute):
As strong acids and bases resp. de-protonate and protonate completely the relationships are very simple:
Acids:
CH=C
Bases:
COH=C
And also for strong bases:
CH=KWCOH=KWC
II. Solutions of weak acids and bases (dilute):
Assume:
CH=x
Then, from the de-protonation reaction equation:
CHA=C−x
And:
CA=x
So:
KA=x2C−x
Reworked:
x2+KAx−KAC=0
This is a quadratic equation with one real, positive root:
x=−KA+√K2A+4KAC2
For example for C = 0.1 mol/L (0.1 M) and K<sub>A</sub> = 10<sup>-4</sup>, we get:
CH=0.003mol/L
This means that only 3 % of the 0.1 M acid has de-protonated!
Note that:
CH≪C
So that:
C−x≈C
And we can apply the following approximation:
KA=x2C
And:
x=CH≈√KAC
In our case (recalculated with the new expression):
CH=0.003mol/L
So:
pH=2.5
So for weak acids:
pH=12(pKA+pC)
Similarly for weak bases:
COH≈√KBC
And:
pH=14−12(pKB+pC)
Here we can draw an important conclusion: where for strong acids/bases the expected oxonium/hydroxide concentrations (resp.) are equal to the
concentrations, for weak acids/bases the expected oxonium/hydroxide concentrations (resp.) are proportional to the square root of the equilibrium
constant multiplied by the concentration.
<hr>
Coming up: Conjugated acids, bases and buffers.
[Edited on 21-1-2016 by blogfast25]
|
|
|
j_sum1
Administrator
       
Posts: 6371
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Nice work Blogfast.
Were you going to discuss what happens with a diprotic acid?
|
|
|
ELRIC
Hazard to Others
  
Posts: 244
Registered: 23-2-2015
Location: Kentucky
Member Is Offline
Mood: No Mood
|
|
Thanks for starting this Blogfast
Will study up after work. You're above post shows "math processing error"
on my screen 24 times though
|
|
|
ELRIC
Hazard to Others
  
Posts: 244
Registered: 23-2-2015
Location: Kentucky
Member Is Offline
Mood: No Mood
|
|
Sorry. It seems to have been fixed after I made that post
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
LaTex does delay things a bit.
Thanks!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Didn't see your post there, for a minute, ooopsie.
In the instalment next to the next one. Ta.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Conjugated acids, bases and buffers:
Consider again a generic weak acid and its de-protonation:
HA+H2O↔H3O++A−
KA=CHCACHA
And:
KW=CHCOH=10−14
It can be shown and is well known from empirical evidence that the:
A−
... ion is itself a weak base. Consider for example how the alkali metal acetates, carbonates, and other alkali metal salts of weak acids form
solutions that are slightly alkaline. This is due to the following protonation reaction:
A−+H2O↔HA+OH−
An equilibrium equation can be defined for this equilibrium:
K∗B=CHACOHCA
Now if we multiply both equilibrium constants we get:
KAK∗B=CHCOH=KW
And:
pKA+pK∗B=pKW=14
A<sup>-</sup> is called the conjugated base of HA (the asterisk points to the conjugation).
Entirely analogously to the above, for a weak base:
B+H2O↔BH++OH−
... the protonated form BH<sup>+</sup> is the conjugated acid of B. An example are the ammonium salts
(of strong acids) which react slightly acidic in watery solution.
Here we can write:
KBK∗A=CHCOH=KW
And:
pKB+pK∗A=pKW=14
Conclusions: the conjugated base of a weak acid is a weak base. The conjugated acid of a weak base is a weak acid. The de-protonated strong
acids and protonated strong bases on the other hand react neutrally.
The sum of the p-values of an acid (or base) and its conjugated base (or acid) always equals 14.
Buffer solutions (principle only):
Consider again the equilibrium constant for a weak acid:
KA=CHCACHA
We can rework this as follows:
CH=KACHACA
Assume we now prepare a solution that contains both the free acid HA at concentration C and its conjugated base A<sup>-</sup> (e.g.
delivered by a Na salt) at concentration C<sup>*</sup>, then we can write:
CH=KACC∗
And:
pH=pKA−log(CC∗)
These are known as the buffer equations.
For:
CC∗=1
Then:
pHbuffer=pKA
Buffers are also frequently prepared from a weak acid and a weak base (not conjugated to the acid).
Buffer solutions have the advantage of having a ‘robust’ pH: pH is not significantly affected by the addition of small amounts of acid or base.
The target pH of the buffer can be manipulated by means of the C/C<sup>*</sup> acid to conjugated base ratio.
<hr>
Exercise:
The pK<sub>B</sub> of ammonia (NH<sub>3</sub> is 4.75.
Estimate the pH of a 0.025 M solution of ammonium sulphate (assume the sulphate ion to be neutral). is 4.75.
Estimate the pH of a 0.025 M solution of ammonium sulphate (assume the sulphate ion to be neutral).
Coming up: Multi-protic acids and mixtures of acids
[Edited on 21-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Multi-protic acids and mixtures of acids
Multi-protic acids can donate more than one proton. Examples that spring to mind are sulphuric acid, phosphoric acid, citric acid, oxalic acid and
maleic acid.
A generic multi-protic acid undergoes a series of de-protonations:
HnA+H2O↔H3O++Hn−1A−
Hn−1A−+H2O↔H3O++Hn−2A2−
And so forth...
With each de-protonation is associated an equilibrium constant:
KA1=CHCH(n−1)ACH(n)A
KA2=CHCH(n−2)ACH(n−1)A
And so forth...
Notice that these equations all share the factor:
CH
Let’s explore the implications of this with a simple example: estimating the pH of a 0.1 M oxalic acid solution.
Oxalic acid is a di-protic, medium acid and we have:
pKA1=1.25,pKA2=4.14
For argument’s sake we’ll tentatively estimate oxonium concentration using the approximation given in a previous post, for a mono-protic acid:
CH≈√KA1C=√10−1.25×0.1=0.075M
For the second de-protonation we have:
KA2=CHCOxCHOx
Re-write this as:
COxCHOx=KA2CH
Or:
COxCHOx=10−4.140.075≈0.001
This ratio shows that the second de-protonation barely happens at all: the presence of oxonium ions from the first de-protonation suppresses the
second de-protonation almost completely (this is in accordance with the Le Chatelier Principle).
Because for most multi-protic acids the subsequent K<sub>A</sub> are mostly several orders of magnitude (factors of 10)
removed from each other (the first de-protonation always having the largest equilibrium constant for self-evident reasons) , treating the multi-protic
acid as a mono-protic one is usually an excellent approximation, except for very strong acids.
<hr>
A more formal derivation of pH e.g. in the case of oxalic acid (or other multi-protic acids) requires the algebraical determination of four variables:
CH,CH2Ox,CHOx,COx
Four variables (unknowns) require four simultaneous equations.
We already have two: both equilibrium equations for the first and second de-protonations.
A third one, with C the nominal concentration of the acid, is a mass balance:
C=CH2Ox+CHOx+COx
A fourth equation is obtained from the neutrality requirement: all positive charges need to be matched with negative ones, in order for the solution
to be electrically neutral:
CH=CHOx+2COx
Together they form a set of four simultaneous, non-linear equations that define the four variables unambiguously. But for reasons outlined above
it’s rarely needed to solve this system of equations and instead a heuristic approach based on reasonable assumptions and common sense usually
suffices to obtain a reasonable estimate of pH, in a much simpler way.
<hr>
Mixtures of acids
The heuristic methodology outlined above for multi-protic acids can easily be extended to mixtures of acids. As long as the difference in equilibrium
constants is (rule of thumb):
ΔpKA>3
... de-protonation of the weaker acid(s) can be considered negligible.
<hr>
Exercise: estimate the NH<sub>3</sub>/NH<sub>4</sub><sup>+</sup> ratio in a solution that is 0.05
M NH4Cl and 0.1 M acetic acid. Data: pK<sub>A,ammonium</sub> = 9.25, pK<sub>A,acetic acid</sub> = 4.76.
Coming up: Acidometry and pH indicators
[Edited on 21-1-2016 by blogfast25]
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
wonderful work up of the topic bf
3 thoughts:
How about some sample problems at various levels from noob to hard core? (I do see a few  ) )
A really basic work up might be less intimidating for the complete novice (eg a simple case study of HCl showing pH change as it is diluted etc)
Someone may be able to contribute a pKa table for some acids?
this will be great for the tutorial subforum discussed in another thread. thx for getting us started
Beginning construction of periodic table display
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This is a very nice treatment of an important subject. Now maybe we can understand more of what Nicodem is about when he talks pKa's. 
Blogfast, you can get paid for this kind of thing you know!
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@diddi:
By all means suggest a 'hardcore' problem and I'll cut my teeth on it.
A table of pK value of common acids and bases was linked to higher up.
@Magpie:
'Real life' teaching, moi? It's my crowd-control powers that would let me down!  (Kermit gets it in the chops!) (Kermit gets it in the chops!)
Thanks both of you for the encouragement!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thread navigation:
1. Water as an acid and a base
2. Weak and strong acids and bases: Acid/base equilibrium constants
3. Essential pH calculations
4. Conjugated acids, bases and buffers
5. Multi-protic acids and mixtures of acids
6. Acidometry and pH indicators
[Edited on 22-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Acidometry and pH indicators
(Cautionary note: this is a fairly basic theoretical treatment of acidometry, not a treatise on the merits of practical titration, down to
which mag stirrer bar to use for which titration!)
A strong base like NaOH completely dissociates in water:
NaOH(s)→Na+(aq)+OH−(aq)
A strong acid completely deprotonates in water:
HA+H2O→H3O++A−
By mixing such solutions, we get neutralisation:
H3O++OH−→2H2O
During the addition of the base to the acid the pH of the solution changes strongly.
If we assume the concentrations of acid and base to be:
Ca,Cb
... and we start from a volume V<sub>a</sub> of acid then if we add a volume V<sub>b</sub> of acid, the
resulting oxonium concentration can be calculated from:
CH≈10−7+CaVa−CbVbVa+Vb
pH=−logCH
At:
CaVa=CbVb
Then:
CH=10−7,pH=7
And before any addition of NaOH solution:
CH=Ca,pH=−logCa
By slow addition of an alkaline solution to an acid solution and monitoring the pH we now have a means of measuring the concentration of the acid
from:
Ca=CbVbVa
This is the basis of acidometry, aka acid base titration.
A typical pH curve for the addition of a strong alkali (like NaOH) to a strong acid is shown below.
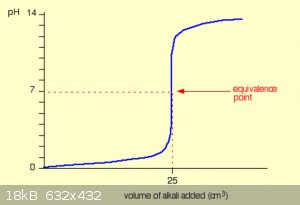
Note that the pH doesn't change very much until you get close to the equivalence point. Then it surges upwards very steeply. This steep surge is easy
to detect.
Titration of a weak acid with a strong alkali
A typical pH curve for the addition of a strong alkali (like NaOH) to a weak acid (e.g. ethanoic acid) is shown below.
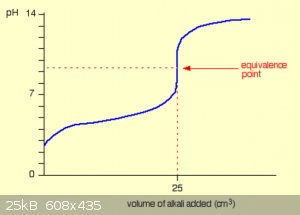
The start of the graph shows a relatively rapid rise in pH but this slows down as a buffer solution containing ethanoic acid and sodium ethanoate is
produced. Beyond the equivalence point (when the sodium hydroxide is in excess) the curve is just the same as that end of the strong acid - NaOH
graph.
pH of equivalence points
A titration is a neutralisation with the formation of a salt solution:
Na+(aq)+OH−(aq)+HA(aq)→Na+(aq)+A−(aq)+H2O(l)
In this post it was shown it was shown that the resulting anion A<sup>-</sup>, or conjugated base, is a weak base.
This means that unlike the titration of a strong acid with a strong base, the equivalence point for a titration of a weak acid with a strong base is
not located at pH=7 but somewhat higher (as the relevant graph also shows).
At the equivalence point the concentration of A<sup>-</sup> is:
CA=CaVA+VB
Using the equation for pH the pH at equivalence point can then be estimated.
Analogously, the pH of the equivalence point will be lower than 7 when titrating a weak base with strong acid.
The position (pH) of the equivalence is important for the selection of the correct indicator.
<hr>
Titration of sodium carbonate:
The titration of sodium carbonate with a strong acid (usually HCl) is an important one because sodium carbonate can be used as a Primary Standard,
allowing to standardise the concentration of acid titrant solutions. It’s also a bivalent base.
Sodium carbonate dissociates completely when dissolved in water:
Na2CO3(s)→2Na+(aq)+CO2−3(aq)
The carbonate ion is a bivalent base, with equilibrium constants:
pKB1=7.7,pKB2=10.4
Two neutralisation reactions take place during titration, perfectly separated from each other due to the difference in pK<sub>B</sub>:
CO2−3+H3O+→HCO−3+H2O
HCO−3+H3O+→CO2+2H2O
A typical titration curve shows the two distinct equivalence points:
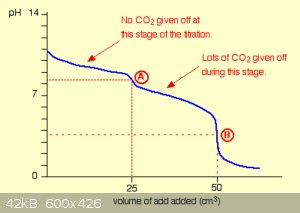
<hr>
pH indicators and equivalence point detection:
Although potentiometric end-point determination has been around for decades, colourful pH indicators continue to be widely used in acidometry.
pH indicators are weak acids that show different colours depending on whether to molecule is protonated or not. I'll represent the protonated and
deprotonated forms resp. as:
HIn,In−
In water, the indicator deprotonates weakly according:
HIn+H2O↔In−+H3O+
and:
KHIn=CH3OCInCHIn
Bear in mind that K<sub>HIn</sub> is really small, typically in the range of 10<sup>-3</sup> to 10<sup>-11</sup>
or so.
We can rearrange the last expression slightly as follows:
KHInCH3O=CInCHIn
Also remember that:
pH=−logCH3O
And:
pKHIn=−logKHIn
We can now see that if:
pH=pKHIn
... the ratio of protonated to deprotonated indicator equals 1! So if both species have different colours, at that pH the colour will be in between
the colours of these species.
But if:
pH<pKHIn
... then the protonated species will dominate and the colour will be that of HIn.
And if:
pH>pKHIn
... then the deprotonated species will dominate and the colour will be that of In-.
In summary we can say that:
pH=pKHIn
... is the turning point of the indicator.
If the turning point of the indicator more or less coincides with the pH of the equivalence point:
pHEP≈pKHIn
... then the equivalence point will be detected accurately.
<hr>
Coming up: Amphoterism
[Edited on 23-1-2016 by blogfast25]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Very nice.
I'm curious, why the preference for CA
over [A]
?
I always found the latter easier to read.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I agree but the 'older' notation is marginally easier to render. And for long 'A' readability does suffer a bit with the bracketed notation, IMHO.
[Edited on 23-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Amphoterism
Species that can behave as acids or bases, usually metal hydroxides, depending on solution oxonium concentration (pH), are called
amphoterics.
Take a generic amphoteric metal hydroxide, usually insoluble in neutral conditions:
M(OH)n(s)
In the presence of oxonium ions a series of protonations can take place:
M(OH)n(s)+H3O+(aq)↔[M(OH)n−1H2O]+(aq)+H2O(l)
[M(OH)n−1H2O]+(aq)+H3O+(aq)↔[M(OH)n−2(H2O)2]2+(aq)+H2O(l)
Up to:
[M(H2O)m]n+(aq)
Note that n and m are not necessarily equal.
So this leads to the solvation of the hydrated M cation.
But in the presence of hydroxide ions the amphoteric species can also form anionic hydroxide complexes :
M(OH)n(s)+OH−(aq)↔[M(OH)n+1]−(aq)
[M(OH)n+1]−(aq)+OH−(aq)↔[M(OH)n+2]2−(aq)
Up to:
[M(OH)m](m−n)−(aq)
Note that n and m are not equal.
So this leads to the solvation of M to a hydroxy-complexed anion.
This also explains why the hydrated M cation of an amphoteric reacts slightly acidic:
[M(H2O)n]n+(aq)+H2O(aq)↔[M(H2O)n−1(OH)](n−1)+(aq)+H3O+(aq)
Some well known amphoteric metal cations include:
Al3+,Cr3+,Be2+,Sn2+,Sn4+,Pb2+,Pb4+,Zn2+,Cu2+,Sb(+3),Sb(+5),As(+3),As(+5)
For aluminium, the distribution of the various species in function of pH for a dilute solution is shown in the following graph:
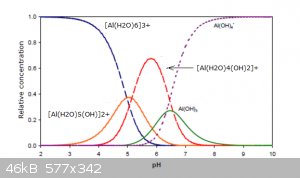
The vertical axis is the relative concentration of the species.
<hr>
Coming up: Enthalpy of neutralisation.
Acids in non-aqueous media and super acids.
[Edited on 24-1-2016 by blogfast25]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Great stuff so far!
However, I think I would be remiss on not commenting on a point for those would be chemist feeling down as their acid's pH is not impressive. Cheer
up, Christmas may not be over yet. Here an extract from some related comments I once did:
Quote: Originally posted by AJKOER  | .......
........
In the case of dilute HCl where the addition of a salt is not problematic for a particular application, one may consider the addition of anhydrous
calcium chloride (or, a concentrated solution thereof) as a possibility. The reason relates to the apparent significant increase in the so called
'activity level' upon adding MgCl2 or CaCl2 or NaCl (in declining order of preference). Here is a real world reference relating to practical
significance in the field of Hydrometallury where leaching out minerals from ores efficiently and cheaply is a major concern. Source: See
"Hydrometallurgy in Extraction Processes", Vol I, by C. K. Gupta and T. K. Mukherjee, page 15 at http://books.google.com/books?id=F7p7W1rykpwC&pg=PA15 .
Note, the author claims there is data confirming that a 2M HCl in 3M MgCl2 or CaCl2 (and also FeCl3) behaves like 7M HCl!
Here is a quote on the matter of discussion in one of the reference sources previously provided by Bfesser on Thermodynamic Activity (see http://en.wikipedia.org/wiki/Thermodynamic_activity ):
"When a 0.1 M hydrochloric acid solution containing methyl green indicator is added to a 5 M solution of magnesium chloride, the color of the
indicator changes from green to yellow—indicating increasing acidity—when in fact the acid has been diluted. Although at low ionic strength
(<0.1 M) the activity coefficient approaches unity, this coefficient can actually increase with ionic strength in a high ionic strength regime. For
hydrochloric acid solutions, the minimum is around 0.4 M.[1]"
[Edited on 22-10-2014 by AJKOER] |
Link: http://www.sciencemadness.org/talk/viewthread.php?tid=38378
So, apparently, even ones dilute vinegar with a good dose of say CaCl2, may be more powerful than you would otherwise expect based on only its pH.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks.
You can find an explanation of the phenomenon here:
http://www.umich.edu/~chem241/lecture11final.pdf
Especially the graph of proton activity coefficient v. solution ionic strength is revealing. Activity coefficient first decreases then increases with
increased ionic strength.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Enthalpy of neutralisation:
Strong acids and bases are completely dissociated in water:
HA(aq)+H2O(l)→H3O+(aq)+A−(aq)
MOH(s)→M+(aq)+OH−(aq)
When these solutions are mixed the neutralisation reaction takes place:
H3O+(aq)+OH−(aq)→2H2O(l)
Note how the ions M<sup>+</sup> and A<sup>-</sup> do not play any part in the reaction: they are spectator
ions.
The neutralisation reaction releases heat (Enthalpy):
ΔHneutr≈−57kJ/mol
So that’s a release of 57 kJ per mol of neutralisation water formed.
Note: don’t conflate or confuse this with the Enthalpy of Formation of water.
This value, for the neutralisation of strong bases with strong acids, is remarkably invariant to the specific acid and base used because the species
are fully dissociated and the spectator ions play no part in the reaction.
In the case of weak acids and weak bases the neutralisation energy can be lower. Since as neither are fully dissociated the neutralisation reactions
can be written as:
HA(aq)+H2O(l)→H3O+(aq)+A−(aq)
B(aq)+H2O(l)→OH−(aq)+BH+(aq)
Added up:
HA(aq)+B(aq)→BH+(aq)+A−(aq)
Among other enthalpies, the dissociation enthalpies of the first two reactions have to be taken into account. With Nernst but without going near
anything like a full derivation we have:
ΔG=−RTlnK
Since as for weak acids and bases:
K≪1
And thus:
ΔG>0
So the dissociations cost energy and that affects the overall enthalpy released during neutralisation.
In one case, the neutralisation of HCN (a very weak acid) by KOH, the neutralisation Enthalpy was found to be as low as -11.7 kJ/mol.
Practical neutralisation exotherms:
The value of – 57 kJ/mol is quite a modest value and for the neutralisation of dilute solutions the effect on solution temperature is usually
negligible.
But for more concentrated solutions the generated heat can be non-trivial and the BP may be reached during mixing.
We can estimate this effect by making a few assumptions/simplifications:
• Assume the heat capacity of the neutralised solution to be that of water and constant over the temperature interval.
• Assume no heat losses to the environment.
The enthalpy needed to heat 1 kg of water from 20 to 100 C is approx.:
4.2×80=336kJ
That requires the following amount of neutralisation water to be formed:
33657=5.9mol
So 1.5 mol of strong alkali and 1.5 mol of strong acid, each dissolved in about 0.5 kg of water will approximately heat the neutralised solution to
BP.
<hr>
Coming up: Acids in non-aqueous media and super acids.
[Edited on 23-1-2016 by blogfast25]
|
|
|
| Pages:
1
2 |