| Pages:
1
2 |
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
What molecule are you talking about Rich_Insane? If caffeine, it's not really proper to call Nitrogens 5 or 7 "amide" nitrogens. Technically, the
functional group is not really an amide, and not really a urea, but it doesn't matter. The only important thing to note is that given the presence of
the carbonyls, the nitrogens have increased SP2 character, and are thus less basic than a tertiary amine. Caffeine can definitely be isolated by
acid/base extraction; I can vouch.
As for arguing what an alkaloid is, who cares. The above discussions of acid/base vs. just base extraction of alkaloids is merely a discussion of how
one would approach the extraction of a basic compound versus an acidic compound. Alkaloid or not, you can use that general approach. If an alkaloid
contains acidic and basic moieties, you're probably best off simply using repeated extraction with organic solvent. Just because a molecule
contains a basic or acidic moiety does not mean it will behave well in an acid/base extraction
That being said it is quite possible that lysergic acid hydrochloride - alkaloid or not - is water soluble. I don't know. But the molecule has so
much other non-polar spinach hanging off the nitrogen that you would be better off extracting with organic solvent.
I don't really know what you're getting at with the alkaloid definition.
[Edited on 28-5-2009 by Arrhenius]
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
The definition states that all alkaloids can be made a hydro(halogen).
If I were to extract an alkaloid, I would if it is polar or not, do extractions first by grinding and washing the material, then adding a polar
solvent (separate the material, depending on if your alkaloid is polar or non-polar), add a non-polar solvent and separate, do an acid-base
extraction, repeat steps, and finally recrystallize.
As for caffeine, I was just guessing the the people above were right. I initially assumed that caffeine had no amide groups. The SP2 is where 2
electrons go to the P shell, forming a hybrid orbital, am I right (I'm trying not to make stupid mistakes)? So the reason the amine is more basic is
because it is a proton acceptor and that nitrogen there in the ring cannot accept protons as well?
The reason there is SP2 is because the Carbon in the Carbonyl shares 4 e with the Oxygen, and in order to bond with the nitrogen, it needs to
hybridize?
If I were doing an extraction i would not go straight base, because the alkaloid may or may not do anything, and would still remain in a whole bunch
of mucilage. I would do solvent extractions, precipitate the proteins with alcohol once the matter is finely grinded, and dissolve the lipids
somewhere in the process with chloroform.
When I was extracting melanin from Shewanella and Aspergillus (which is slightly different from plants, but there are similarities), I did at least 5
freeze-thaw cycles to break down the cells, which is extremely important. I would do this for a few hours, then I did a quick centrifuge. I'm not sure
if that's feasible, but it's not too hard to obtain a cheap centrifuge (microcentrifuge). Then I vortexed the mixture. After that I added chloroform
to dissolve lips from the cell wall. Centrifugation yielded this debris. Keep a cycle of this. Then add isopropanol under heat to cause proteins to
precipitate and denature. I'm not sure how well this applys toplants; You want to destroy the cellulose cell wall, so you need something for that.
The above only applies, however if the alkaloid is intercellular. If it is found floating around in the vascular tissue, then you have to ensure a
crude cellular destruction and dissolve proteins and lipids (then proceed with acid/base, polar/nonpolar extractions).
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
The reason the "amide" and "imide" nitrogens are non basic is due to the canonicals that can form, involving the carbonyl group. The lone pair on the
nitrogen is delocalised onto the carbonyl, thus reducing the ability as a base; thus amides are essentially neutral.
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
What do you mean by canonical?
So what you are saying is that the lone pair on the nitrogen is distributed in the whole nitrogen-carbonyl group thus disabling the Nitrogen's ability
to accept the proton?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
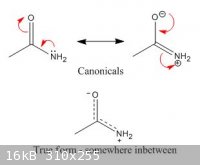
Thats exactly what I am saying. A canonical is a resonance form, but the *true* structure is neither canonical, merely somewhere in between (as we
don't know for certain).
Look under "amide properties" on this page:
http://en.wikipedia.org/wiki/Amide
[Edited on 28-5-2009 by DJF90]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm curious about two things: is coffee acidic or basic? I've never checked but CO2 is acidic and does a good job extracting caffeine from unroasted
beans in supercritical state.
What does "kewl" or "kewlish" mean??
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
chemrox: The term kewl is discussed somewhere on the forum; UTFSE. Alternatively check the urban dictionary (just google "urban dictionary").
Coffee is a chemical mixture; it may contain both acidic and basic components. Caffeine however, the main alkaloid in coffee, should be basic by my
reasoning. Supercritical CO2 I dont think is acidic; I believe its utilisation to extract caffeine from coffee beans comes from its solvent abilities
(just like how ethanol is hardly basic, but is a better solvent than water for many organic compounds).
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
DJF90: I've never heard the term 'canonical' in US chem books. I presume, from your drawing, that it's the same as a 'resonance form' or 'resonance
structure. You're correct about the real structure being somewhere in between everything we can draw, but I do believe high level quantum mechanics
can predict electronic structures quite well.
Chemrox: Coffee is acidic from tannic acids and phosphorus acids (supposedly).
Rich_Insanity: DJF90's explanation of why it has SP2 character looks pretty good. Ya, centrifugation works miracles with plant extractions. I don't
think you necessarily need to break apart cell walls to extract chemicals from within. Sometimes breaking up plant material too much just gives a lot
of chlorophyll in the extract. I've even used aqueous acid refluxes to facilitate extractions. There really are lots of options. Very seldom can
you crystallize a desired product from an extract though; at least not as a pure product.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Arrhenius: Yes I believe it is another way of saying the same thing. Look at this following link under the heading "Resonance" (I believe this is a
page from MSU - Michigan state university if I am not mistaken, meaning that this is not just a UK term as I had once thought):
http://www.cem.msu.edu/~reusch/VirtualText/intro3.htm
Having the chlorophyl extracted with the desired material I imagine is quite problematic (at the very least a pain in the arse). If I remember
correctly though, chloroform is useful for solvent extractions because it extracts only a minimal amount of chlorophyl. At least I think it was
chloroform.
Generally for obtaining a crystalline product, the organic extract is dried with a drying agent, filtered into a flask and the solvent removed either
by distillation or by use of a rotory evaporator. Once most/all of the solvent is removed, if crystallisation hasnt started yet it can be initiated
using an ice bath and "scratching" with a glass rod. The crystalline precipitate can then be removed from the flask and recrystallised 2-3 times to
yeild a pure product.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Caffeine, for example, does not form hydrochlorides in aqueous solutions since it is barely ten times more basic than water which essentially means
that the solvent more than efficiently competes for the protons and if you remove the solvent the HCl will be removed as well. I think it forms some,
though unstable, "salts" from diethyl ether, kind of like some amides can. I do not know where anybody got the idea that caffeine can be purified
using an acid extraction - none of its lab or industrial extractions use any such procedure.
Rich_Insane, if you would have read my previous post you would have noticed that alkaloids need not to be basic. Colchicine is completely neutral and
yet it is a classical alkaloid. Morphine has the pKa of 9.9 in its neutral form and is thus a weak acid even when the nitrogen is not protonated (even
though an acid it is still considered as one of the most classical alkaloids!). This essentially means that morphine can be extracted from a nonpolar
solvent using aqueous NaOH, like you would otherwise extract an acid.
PS1: The names or the orbitals are in low caps. There are no "S", "P" or "SP2" orbitals, only "s", "p" and "sp2" orbitals.
PS2: "Canonical" means something similar to formalistic, that is the minimum not yet completely false form of something that is otherwise too complex
to be described to fit the reality. The canonical forms are determined by conventions and rules rather than by the models fitting the reality. In
reality chemical structures are always canonical except when determined by single crystal XRD and even then they are only real for the structure the
compound has when crystalline. The term "canonical" is an ubiquitous term in science and mathematics: http://en.wikipedia.org/wiki/Canonical
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Caffeine is extracted by supercritical CO2 or a nonpolar solvent (methylene chloride) (there are other methods, but as far as I know these are the
main extraction methods.
It depends on what alkaloid you are working with. There are several classes of alkaloids, since it is a broad category.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
What about taking grinded coffee and soaking it in dillute HCl to dissolve caffeine from it as caffeine hydrochloride, filtering it and then adding a
base to make caffeine again water insoluble? Would that work?
|
|
|
| Pages:
1
2 |