| Pages:
1
2 |
koopatroopa023
Harmless

Posts: 6
Registered: 22-5-2009
Member Is Offline
Mood: No Mood
|
|
Straight to base extractions
When extracting alkaloids from plant material, I understand that it is usually gone about by an acid base extraction. I also know that straight to
base extractions work just as well, however I do not understand how this process works without using the acid. Could someone please enlighten me?
Thanks!
|
|
|
pHzero
Hazard to Self
 
Posts: 89
Registered: 16-5-2009
Member Is Offline
Mood: Fully substituted
|
|
Erm I'm not entirely sure of the sciencemadness rules, but I don't think drug talk's allowed here.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Alkaloids arent always "drugs" pHzero... take caffiene for instance.
I dont see how just a basic extraction will yield your alkaloid. Normally acid is added to the organic extract, forming the ionic "ammonium" salt,
which is extracted into the aquous layer (where ionic compounds usually reside). The other non-amine compounds stay in the organic layer which is
discarded. Then base is added, forming the free amine, which is insoluble in water, but soluble in an organic solvent (ether is usually a good
choice). The amine can therefore be extracted either by filtration of the precipitate (the amine), or adding an organic solvent such as ether and
discarding the aqueous layer. The organic extract can then be dried, filtered (to remove the drying agent) and evaporated to yeild the amine.
|
|
|
Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
pHzero, please get your facts right before accusing people of drug-preparation/extraction. Alkaloids, as DJF90 has said are not always drugs or drug
precursors.
We stand in the same field of thought as DJF90- the principles of alkaloid extraction are not present. We do have a copy of a book entitled: 'The
Plant Alkaloids' which we are happy to post, however tropane ring alkaloids as well as iso-quinoline alkaloids are discussed, hence permission from a
moderator will need to be given first as we aren't sure whether or not it will be appreciated.
Not having read the entire book, we aren't sure how much use it will be, but its one more potential reference.
|
|
|
pHzero
Hazard to Self
 
Posts: 89
Registered: 16-5-2009
Member Is Offline
Mood: Fully substituted
|
|
Ok, sorry for making assumptions. It just seemed strange that someone should join the board and their first post be asking how to extract alkaloids
without explanation - that, i think, is how morphine's extracted from poppies to make heroin...
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yes and also how cocaine and other alkaloids are extracted. However the original poster made no reference to any specific compound and so we have to
assume he is not up to mischief.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by DJF90  | | Yes and also how cocaine and other alkaloids are extracted. However the original poster made no reference to any specific compound and so we have to
assume he is not up to mischief. |
Even if he wanted to know about the extraction of cocaine from the plant material, so what? It is probably already well documented in scientific
journals, does it become "mischief" when the ordinary citizent want to see the information? Well, he should have googled before posting, but that's
another issue.
Caffeine is a drug, it is a CNS stimulant, as such it is addictive, you can read more about its mechanism of action here: http://en.wikipedia.org/wiki/Caffeine
[Edited on 22-5-2009 by Sandmeyer]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Sandmeyer: You're misunderstanding what I said. There is a reason for the quote marks...
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
This might be possible if the alkaloids is in the form of salt in the plant itself, forming ammonium ions with organic acid.
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Pomzazed: In this case if you try to do the extraction with an organic solvent then you'll get everything but the alkaloid. If instead you extract
with water, then theoretically the ammonium salts will go into solution. A base can then be added to cause precipitation of the free amine.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | | Pomzazed: In this case if you try to do the extraction with an organic solvent then you'll get everything but the alkaloid. If instead you extract
with water, then theoretically the ammonium salts will go into solution. A base can then be added to cause precipitation of the free amine.
|
Most extraction of alkaloids start with extracting the dried biological sample using an organic solvent, most commonly methanol or ethanol. Salts of
alkaloids are generally well soluble in lower alcohols. Another method to treat the biological sample is to extract it with chloroform or other such
solvent with the addition of a couple % of conc. aqueous ammonia. This crude extract is then treated by one of the many separation techniques like
extractions, recrystallizations, chromatography, etc..
Anyway, there are numerous examples one can find in the literature and without knowing what (type of) alkaloid(s) extraction is being discussed any
guesswork discussion in this thread is complete nonsense. Besides, what is "straight to base extraction" supposed to mean anyway? That is why I'm
moving this thread to the Beginnings section just like I do with any such lazily and vaguely formulated questions posted without a single reference. I
still do not understand why people insist in asking questions posed in such a way as to remain non-answerable?
PS: About this topic of whether discussion about drugs is allowed or not... I do not even understand where the doubt arises. About 30% of all thread
in the Organic chemistry section is either directly or indirectly connected with drugs, legal or illegal (this is not a law forum so we make no
distinction). The confusion might arise from the forum policy that does not allows kewlish, profit related or lazy posting. For some reason about 90%
of those interested in drugs, and about 99% of those interested in illegal drugs, are either kewls, demented, lazy and/or greedy, which is why drug
related post often end up in Detritus (not due to the topic itself). Threads where drugs are discussed coherently in a scientific manner are welcome.
The same policy is valid also in the Energetic materials section.
pHzero, you are new here and you obviously know less about the forum culture than most of others who had more experience. So, next time you have
doubts, use the post report button and/or leave it to the moderators.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
22-5-2009 at 23:30 |
koopatroopa023
Harmless

Posts: 6
Registered: 22-5-2009
Member Is Offline
Mood: No Mood
|
|
Hey everyone, thanks for trying to be so helpful. Perhaps I should be more clear on my line of thought. Take for example the extraction of
Dimethyltriptamine from various plants. I am not trying to make this specifically about illegal drugs, but this is the only example I can think of at
the moment. I have read that simply adding a base to water up to a pH of around 9-13, and then adding that to plant material will work just as well
as acidifying and then adding the base to raise the pH back up. I found this strange that this would work without acidifying, and I thought I'd ask a
more academic board than to seek elsewhere for answers that would only pertain to that specific alkaloid. It works, but I do not fully understand why
this can be done. I assume that this is not some singular phenomenon pertaining to only this one alkaloid, but would also work for all alkaloids and
possibly other compounds found in various plant materials. Thanks so much for any help with grasping why this can be done.
|
|
|
koopatroopa023
Harmless

Posts: 6
Registered: 22-5-2009
Member Is Offline
Mood: No Mood
|
|
Hey back again, here's the two extractions of the same compound, but one with the standard acid-base extraction process, and the other with the
"straight to base" process
Acid base extraction: http://www.erowid.org/chemicals/dmt/extraction_guide1/dmt_ex...
Straight to base extraction: http://www.erowid.org/plants/mimosa/mimosa_chemistry1.shtml
Hopefully this will help people understand where my predicament is. Both of these work, I just don't see how the straight to base extraction does.
Any help understanding would be wonderful. Thanks again.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
The 'straight to base' extraction would probably not be the first choice of a professional desiring to isolate plant alkaloids. By definition, an
alkaloid contains atleast one amine nitrogen and an aromatic ring. It's all a matter of what you're extracting your alkaloid away from.
If you throw in a bunch of base, grind up whatever plant, and extract with a non-polar solvent, you'll extract basic compounds (such as alkaloids) and
acidic compounds (such as tannins, etc.) will remain in the basic aqueous layer. You will also extract neutral compounds into the organic layer by
this procedure. However, if you extract your plant into dilute aqueous acid, wash with organic solvent (removes neutral + acidic compounds, e.g.
carboxylic acid containing molecules, tannins, etc.), basify the resulting aqueous fraction to pH>10 and extract with organic solvent, this final
organic extract will contain ONLY (theoretically) basic compounds such as your alkaloids, and fewer neutral compounds.
|
|
|
koopatroopa023
Harmless

Posts: 6
Registered: 22-5-2009
Member Is Offline
Mood: No Mood
|
|
Thank you Arrhenius, that was quite informative. Now my question is if you don't acidify first does the compound go straight to freebase form in the
basic aqueous layer? Or does it somehow go into its salt form and then its freebase form before going into the organic layer?
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Alkaloids probably exist in both salt form(many possible counter ions) and as free bases in their source plant etc. Technically, if you put them in
a neutral solution of water, most will be protonated as +HNR3 or +H2NR2 ammonium salts with a hydroxide counterion. By adding excess base, it pushes
the equilibrium towards NR3 or HNR2 species (free base) which is organosoluble.
|
|
|
koopatroopa023
Harmless

Posts: 6
Registered: 22-5-2009
Member Is Offline
Mood: No Mood
|
|
Much appreciation Arrhenius, that really helped me grasp what was happening.
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
Arrhenius;
Thanks for posting that. It was basically what I tried to say but at the time i posted I was a bit sneezy and may posted a not-quite-comprehensible
sentence.
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Most alkaloids aren't drugs.
I will use an alkaloid that is a precursor for this example, but only as an example (and I may be wrong).
Lysergic acid is an alkaloid extracted from the ergot fungus. Since it is a carboxylic acid, it will react with a base, forming a salt. This salt is
water soluble. So the answer yes, but if you are working with an acidic alkaloid. And if you want a really pure product, you probably have to go
further, and do more research. I know that some people do a solvent extraction with methanol, then dichloromethane, then do the acid base extraction.
Many alkaloids are extracted with kerosene, like cocaine, and others.
Many plants already have the salt of the alkaloid already (as Arrhenius said).
Keep in mind however, that not all alkaloids are amine-containing. Caffiene does not contain amine, but just nitrogen. And some extracts from plants
have no nitrogen at all, but that's a different matter.
You have to do some research more or less on the structure of your alkaloid.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Caffeine has TWO tertiary amine nitrogens. Granted, they are part of a ring system, but that does not stop them from being amines.
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Sorry, I meant it didn't have any primary or secondary amine groups.
Some terpenes are not amines, they are fully carbon and oxygen sometimes.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The structure of caffeine does not have any amino groups. It has 4 nitrogens, two of which are amide nitrogens, one is an sp2 hybridized nitrogen, and
one has its lone electron pair incorporated in an aromatic resonance system; but it has no amine nitrogens. Check its structure: http://en.wikipedia.org/wiki/Caffeine
Lysergic acid is not an alkaloid and it is not extracted from fungi/plant material. It made by hydrolysis of ergot alkaloids. The definition of what
is an alkaloid and not is part of convention, not about chemical properties. The only thing that alkaloids need to be, is to be present in living
organisms, to contain at least one nitrogen and not to be already classified into other groups (for example, amino acids or nucleic bases can not be
called alkaloids because they are already classified otherwise). Therefore, alkaloids need not to be amines, they can be anything from pyridines to
amides, but always containing nitrogen. It thus also true that alkaloids need not to be bases. For example, the pKa of caffeine is about 0, which is
barely more basic than water. Colchicine is even less basic, and so on. Morphine is even a base and an acid at the same time. Even the wikipedia entry
for alkaloids (http://en.wikipedia.org/wiki/Alkaloids) is in conflict with itself. It claims that alkaloids are "naturally occurring chemical compounds
containing basic nitrogen atoms" yet it gives caffeine as an example of an alkaloid!
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Bromide
Harmless

Posts: 24
Registered: 20-6-2007
Location: Flavor country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | | Caffeine has TWO tertiary amine nitrogens. Granted, they are part of a ring system, but that does not stop them from being amines.
|
You sure you didn't mean to write that caffeine has THREE tertiary amine nitrogens? 
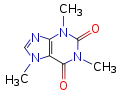
|
|
|
TIETSE
Hazard to Self
 
Posts: 53
Registered: 21-8-2008
Member Is Offline
Mood: No Mood
|
|
koopatroopa023 look for this book:
The Technology and Chemistry of Alkaloids
de Frank E. Hamerslag - 1950
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
I'm sorry, I forgot all about the fact that lysergic acid is from Ergotamine. However, i am sure there are carboxylic acid alkaloids, I just can't
think of any off the top of my head. I would think that those Nitrogens are just Nitrogens in a ring (like Pyridine, which has 1). The two on the
Benzene side of the ring are amides, am I right? Alkaloids were a generic term given a while ago to substances found in plants that could be used
medically. So the current definition from dictionary.com is: Any of a large class of organic, nitrogen-containing ring compounds of vegetable origin
and sometimes synthesized, some of which are liquid but most of which are solid, that have a bitter taste, that are usually water-insoluble and
alcohol-soluble, that combine with acids without the loss of a water molecule to form water-soluble hydrochlorides, hydrobromides, or the
like, and that usually exhibit pharmacological action, as nicotine, morphine, or quinine.
|
|
|
| Pages:
1
2 |