| Pages:
1
2
3
4
5
..
37 |
DBSP
Harmless

Posts: 10
Registered: 17-9-2002
Location: sweden
Member Is Offline
Mood: No Mood
|
|
I have some questions about DPPP, what solvents is it suluble in? Can it be used in metal caps etc.
I'll probably try to synthesise it this weekend if I have eneugh time using 30% HCl, 40% H2O2, pure acetone.
Does more concentrated chems increase the yield in this process or does it lower it because of the larger ammounts of heat generated ?
|
|
|
BLAST_X
Harmless

Posts: 29
Registered: 21-9-2002
Location: USA
Member Is Offline
Mood: No Mood
|
|
I`cant say the correct density of DPPP
(it exist nowhere information about this compound)
and i don`t have equipment to measure it exactly.
When you calculate, H2O density = 1,0, and DPPP are very light crystals,
[big molecules and high molecular mass, C18H26O2(O2)5],
which going on the surface of the water (AP = density ~ 1,18 [> 1,0] sinking to the bottom),
density of DPPP is < 1,0.
I think density is 0,6 –0,8 g/ml ?
This low density are opposing to the high explosive velocity of DPPP (~9000 m/s)
and similar explosives with vod`s > 8000 m/s (octogen, d ~ 1,9 g/ml).
The most of high explosives with vod`s
> 8000 m/s have a density > 1,6 g/ml.
A another example for low density is tetracene (2,3-naphthacene, 2H8N10O):
d ~ 0,45 g/ml and vod > 4500 m/s.
You can say rough, higher density = higher vod.
(oxygen balance are important also !)
for instance: (vod`s at this density)
octogen, C4H8N8O8:
d ~ 1,9; vod ~ 9100 m/s
tetranitroglycoluril, C4H2N8O10:
d ~ 1,98; vod ~ 9200 m/s
dinitroazofuroksan, C4N8O8:
d ~ 2,0; vod ~ 10k/s
I`ve tested pressed DPPP in a blasting cup without a primary igniter.
A warning before !
When you test a explosive don`t use any metal pipes, cups or matches, with new or unknown substances - under no circumstances !!!
The explosive can form ultra instable isomers and substances with the metal
and the air, which can explode only with the presence of sun-light or with a
touch !!! (look at picric acid or HMTD)
I make safe blasting cups and matches with paper and plastic only, when i test a explosive.
I`ve pressed a charge of 50 g DPPP in a 5 mm hard-paper cup (90x70 mm) careful to high density with a big glas-rod.
To set off the charge, i`ve put a match inside the cap.
A little paper-pipe (3x3x50mm), filled with a fine powdered mixture of KMnO4 + S
in a ratio 1:1.
(look at attachment)
The better way to set off DPPP is certainly electrical, with high temperature without the presence of O2.

all you need is C - H - O - N
|
|
|
Physics
Harmless

Posts: 2
Registered: 25-9-2002
Location: Russia, Kaluga
Member Is Offline
Mood: No Mood
|
|
Phorone
The sirs, you tried to bleed phorone in the pure state? That if to pour out an intermixture acetone / HCl in a great many of water. (Phorone in water
we shall not dissolve)
P.S. Excuse me for mine English.
|
|
|
BLAST_X
Harmless

Posts: 29
Registered: 21-9-2002
Location: USA
Member Is Offline
Mood: No Mood
|
|
Fact is, the aceton reacting with H2O
(if you have 20% or 35% HCl, H2O is always present, which are required for the synthesis)
and HCl as a catalyst to a soluble form of Phoron, which don`t precipitate from the solution !
(look at attachment)
I think, when you moderate the reaction in this direction and the Phoron precipitate from the solution,
the synthesis of DPPP fails.
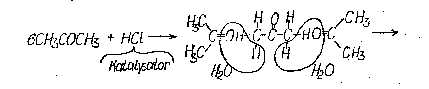
all you need is C - H - O - N
|
|
|
Physics
Harmless

Posts: 2
Registered: 25-9-2002
Location: Russia, Kaluga
Member Is Offline
Mood: No Mood
|
|
phoron
I hoped already at the first stage (deriving of phoron) to eliminate any opportunity of synthesis AP. Therefore I want to receive pure phorone
(without an impurity of acetone). Certainly after that I again to phoron shall add HCl.
|
|
|
BLAST_X
Harmless

Posts: 29
Registered: 21-9-2002
Location: USA
Member Is Offline
Mood: No Mood
|
|
The presence of AP or a other dangerous explosives or peroxide in form of any impureness in the main-product are
a bad thing always.
I think, when everybody make a explosive, it`s dangerous always, but nobody should ignore all the warnings and all the descriptions of them !
When you make AP or a other sensitive peroxide especially.
Everybody see the simple procedures and the high yields of peroxide-synthesis, but the dangerous side are ignored.
Look at the web, how many sites describe the production of more dangerous substances,
for instance:
glycerol tri/tetra-nitrate , AP or picrates
in large amounts.
It`s irresponsible !
Can you conceivably isolate DPPP with adding pure Phoron to a mixture of HCl/H2O2 ?
It`s a expensive way certainly.
I think the better way are, find out a correct possibility to purificate DPPP and destroy all traces of AP correctly.
all you need is C - H - O - N
|
|
|
BLAST_X
Harmless

Posts: 29
Registered: 21-9-2002
Location: USA
Member Is Offline
Mood: No Mood
|
|
Try out it, Physics.
You can buy some Phoron
at Sigma-Aldrich,
2,6-dimethyl-2,5-heptadien-4-one, Diisopropylidenaceton
(CH3)2C=CHCOCH=C(CH3)2
138,2 g/mol
mp = 28,1 C
bp = 198 C
d = 0,9
CAS Number: 504-20-1
Merck Index: 12,7488
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
If you isolate the phorone crystals and remove any trace of organic impurities then it should yeild relitively pure DPPP. So i don't quite see what
your problem is.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
beilstein
From Beilstein:
TITLE Catalytic Cyclocondensation of acetone to isophorone
AUTHORS Ramanamurty, K. V.; Salvapati, G. S.
SOURCE Indian J.Chem.Sect.B 1999, 38: 1 24 - 28
DOCUMENT TYPE Journal
CODEN IJSBDB
LANGUAGE EN
CNR 6208963
ABSTRACT Cyclocondensation of acetone involves aldol condensation leading initially to the primary condensation products (PCP), diacetone alcohol and
mesityl oxide, which again react with acetone to form phorone, isophorone, mesitylene, etc. Relative selectivities of chromia, γ-alumina,
magnesia and calcium oxide catalysts have been studied in a flow reactor at 360-520°C. Alumina and magnesia are found to favour isophorone formation
at 360°C with 45 % selectivity but the selectivity steeply falls with the increase in temperature to 520°C due to rise in decomposition products.
Primary condensation products are formed more with magnesia; selectivity to mesitylene is more with CaO (ca. 66 % at 400-480°C) and Cr2O3 (50-55 % at
480-520°C).
COPYRIGHT Copyright © 1988-2001, Beilstein Institut für Literatur der Organischen Chemie licenced to Beilstein Chemiedaten und Software GmbH and
Beilstein Informationssysteme GmbH. All rights reserved
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
BLAST_X
Harmless

Posts: 29
Registered: 21-9-2002
Location: USA
Member Is Offline
Mood: No Mood
|
|
I don`t have any problem with this.
The question are, be worth the high expenditure, first makeing pure Phoron and at next DPPP, when you use DPPP as a explosive ?
It`s a big rubbish, adding aceton to HCl
to isolate pure Phoron.
The yield are definitely very low.
The major product is this polymerized Phoron
C9H18O3,
and minor product the solid Phoron.
C9H14O.
I`ve read a desciption in a other forum,
to isolate Phoron with high yield in a aldol condensation process in presence of a alkali catalyst and under reduced pressure.
for instance:
http://wngr343a.chem.orst.edu/~gablek/CH336/Chapter18/Aldol....
I dont`t know, it`s a realistic possibility
with a home-lab equipment ?
Everybody should decide the method by itself.
all you need is C - H - O - N
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
Seperating AP from DPPP
I remind it being mentioned that phorone dissolves in alcohol; to me it´s very likely that DPPP also dissolves in alcohol, just very similar to other
substances and its derivates.
If it does, there would be the possibility to seperate DPPP from AP, which i hope, is not or only badly soluble in alcohol.
HLR
10 fingers present.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
Measuremt of density without pyknometer
The most simple method of measuring the crystal density of a water-insoluble substance is to prepare salt solutions and then test wether the produced
crystals sink or swim.
An example: prepare a saturated solution of ammonium nitrate, lead nitrate(i tried this and got a solution with a density of about 1,3g/ccm), sodium
chlorate or whatever that has a very good solubility and has a high density itself.
The only thing to take care of is to use a measuring flask and a balance that provides good accuracy.
If this is a problem, prepare bigger volumes of solution to minimize the error.
I seek for other salt solutions that perform densities of near 1,5g/ccm and above....
HLR
10 fingers present.
|
|
|
BLAST_X
Harmless

Posts: 29
Registered: 21-9-2002
Location: USA
Member Is Offline
Mood: No Mood
|
|
When DPPP or a peroxide dissolves in ethyl alcohol (aceton ?) and it recrystallizing out from the solution by adding H2O, the quality are bad
(crystal-structure, clumpy).
As long as no better possibility exist to handle safe with the crude product, it should been so.
all you need is C - H - O - N
|
|
|
DBSP
Harmless

Posts: 10
Registered: 17-9-2002
Location: sweden
Member Is Offline
Mood: No Mood
|
|
I found this at E&W, might be interesting, not shure though.
http://www.rsc.org/CFCart/displayarticlefree.cfm?article=8%2...
You'll probably have to copy/paste
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
breakthrough in sight?
I want to give an impuls for pushing our research:
We have the patent, which is vague for many parts of the synthesis, sure.
www.sciencemadness.org/library
(just to remind anyone: we HAVE a small fine library which is up to grow...)
But we also made several experiments showing that a water-insoluble substance, pure or impure, in any case not containing acetone any more, thus not
contaminating the final product of peroxidation with acetone peroxide, can be obtained by heating a mixture of (50ml conc. HCl and 50ml acetone)
acetone and HCl for several hours (ideally under reflux) and following neutralization with sodium (bi)carbonate.
It was important not to exceed pH7 by adding too much sodium carbonate or NaOH..., cause it turned out the product re-dissolves not only under acidic
conditions but also under strong alcaline conditions.
It has been noted by all forum members who tried to make phorone using this method, that some decomposition that leads to carbon traces in the
solution, giving it a dark colour, took also place.
I also tried this and found the final yield of the product to be very low.
A 100ml batch of acetone/HCl resulted in approximately 1-2g.
To get a more pure product, the phorone could be recrystallized from ethyl alcohol(this is not yet tested).
But from my point of view the low yield hasnt a big impact cause the two substances are really dirt cheap, and the later product DPPP is supposed to
be used only in small quantities in blasting caps and the like.
The next step should be obvious: dissolving the dried, washed product in HCl to give the chlorinated phorone.
To get a higher yield in the peroxidation step i think it would be better if the pentachlorphorone(by-products excluded here, lets be optimistic...)
would be (very carefully!) dried before the final peroxidatoin step.
I think the chlorinated phorone will be a polar substance(due to the high electronegativity of chlorine and the geometry of the molecule), thus better
dissolving in 30% aqueous H2O2.
The amounts used in the combined chlorination/peroxidation step can (as always) be played a lot with, only considering the equations from the german
patent i calculated the following:
C9H14O + 5HCl + 5H2O2 > C9H13OCl5 + xH2O + yH2O2 > 1/2 DPPP(precipitates) + 5HCl + soandsomuch H2O
(the patents states that nascent chlorine is needed for the chlorination step, so it is actually more than for the peroxidation alone)
From this you can calculate the mass of phorone and the needed volumes of conc. HCl(density is 1,3g/ml) and 30% H2O2 (also 1,3g/ml).
I might have some mistakes in my equations, as this is always possible, and im really really tired.
Give me some feedback.
Could someone calculate this to the end?-I wont make it this time, zzzzzzzzzz
HLR
10 fingers present.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
please ignore the part of isolating pentachlorphorone before the peroxidation step, i think this is not really necessary.
I forgot to delete it.
thx
10 fingers present.
|
|
|
BrAiNFeVeR
Harmless

Posts: 42
Registered: 23-5-2002
Location: Belgium
Member Is Offline
Mood: busy
|
|
Well, the sublimation method does NOT work :-(
It's been lying in my lab for more then a month now, and it still smells like AP.
And on quantity ...
There's about 1 gram left of a starting batch of 5 grams !!
So yields are definately not worth having such an amount of sensitive primary lying around for more then a month!!
|
|
|
bonemachine
Harmless

Posts: 47
Registered: 27-10-2002
Member Is Offline
Mood: No Mood
|
|
I am ready to try this method anyone had any results?
|
|
|
bonemachine
Harmless

Posts: 47
Registered: 27-10-2002
Member Is Offline
Mood: No Mood
|
|
-
I am just ready to make this compound does anyone had any good results?
|
|
|
bonemachine
Harmless

Posts: 47
Registered: 27-10-2002
Member Is Offline
Mood: No Mood
|
|
Forgive me for the double post i thougt the first didn't worked
|
|
|
bonemachine
Harmless

Posts: 47
Registered: 27-10-2002
Member Is Offline
Mood: No Mood
|
|
Ok i made a 50/50 solution of HCL and acetone and it heats up quickly. After a wile it forms small crystals at the sides of the container. After being
cool i added the H2O2 and now i am waiting.
Is there anything specific about the aperance of DPPP so i can recognise it? Does it have for example a diferent colour or crystal size than AP?
excuse me for bad english.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Reactions of acetone with HCl
I spent two weekends doing this....
1) A) A batch consisting of 30mL 30% HCl and 20mL of commercial acetone in a loosely stoppered flask was heated for several hours until the liquid had
a golden yellow colour.
The remaining HCl was neutralized by addition of solid sodium carbonate and i recognized that a yellow-white pptate formed while the liquid cleared
up.
The pptate was filtered off and washed with about 500mL of cold water.
Then i dried the pale yellow to white pptate on a water bath(it was actually steam) and weighed it.
Yield of product was 1,5 g.
The product was insoluble in any of the common organic solvents(etOH, hot H2O, acetone, xylene, petrol...).
Soluble in dilute HCl and dilute H2SO4 forming a deep red solution.
B)The same mixing proportions and volumes with extended reaction time.
(Deep red colour of liquid.)
The above process was repeated and 1,7 g of the product were obtained.
C)The same again.
Waited just more till the liquid was a deep dark red, like a blood conserve.
Yield was again 1,7g.
Funny: the powder seems to be an acceptable pH indicator for pH 7: being dark to light red in acidic media, decolourizing at pH 7.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
1) 25mL of 30% HCl were added to 75mL of acetone and treated like in C) of the previous post.
Yield was 1,6 g.
2)25mL of dilute H2SO4 with HCl (1:1)were added to 75mL of acetone and kept for 3h on a heating plate at approx. 60°C.
Approximately 60mL of the batch remained due to the loss of acetone by evaporization.
The mix had a light orange-red colour and yielded 0,6g of the abovementioned product.
3)25mL of H2SO4*6H2O(dilute sulfuric acid, saturated with water) were given to 75mL of acetone and heated as above for 3h until the solution had a
golden yellow colour.
Yield was 0,2g of a similar product in solubility and optical appearance.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Interesting. Those yields are terrible, though. Obviously 90% yields aren't attained as easily as the patent would have us think. Are you going to try
forming the peroxide, or try to find a better way to make phorone first?
I've searched the ACS journals for information about condensation reactions of acetone to phorone, but the main references are old (late 19th century)
and often in German.
|
|
|
DBSP
Harmless

Posts: 10
Registered: 17-9-2002
Location: sweden
Member Is Offline
Mood: No Mood
|
|
I would just like to point your attention to something an E&W member came up with in the "holy grail organic peroxide" thread.
He might just have a point there.
http://www.roguesci.org/ubb/ultimatebb.php?ubb=get_topic;f=2...
Have a look at the bottom.
|
|
|
| Pages:
1
2
3
4
5
..
37 |