| Pages:
1
2
3
..
37 |
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
Diphoronepentaperoxide (DPPP)
Did anyone hear of this compound?
It´s mentioned in german patent DE1951660, and is an organic peroxide that is said to be as brisant as HMX(Vdet=9000m/s) and is surprisingly made of
the same things as AP: HCl, H2O2 and Acetone.
First i thought it was a fake, but then i found out the patent really exists...
HLR
|
|
|
kingspaz
Hazard to Self
 
Posts: 55
Registered: 23-7-2002
Location: UK
Member Is Offline
Mood: No Mood
|
|
or the patent is a fake...
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
No!
I have found the patent on the german patent server. Here is a structure I made.
The only problem is that i'd expect the double bonded C's to accept a peroxide bond, but then they can't be double bonded anymore and the other C has
to accept a H.

One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I'm wrong!
I made some severe failures in that molecule...please ignore it..I'm working on a new one
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Got it
Finally figured out the correct structure.
The left one is the one according to the patent I found. But I feel it must be more like the left one, because I don't see any explanation why the
other 2 methyl groups wouldn't form a peroxide bond also... HELP! Philou!
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
AAAAAAAARGH
Somehow the picture didn't come along....
Sorry for clogging the thread up...
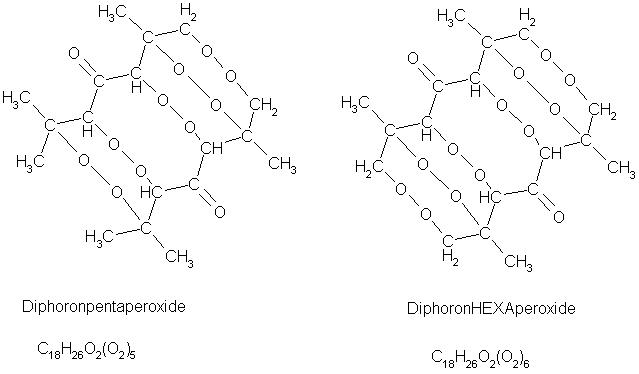
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
You did a nice job with the jpg....i think i will print that interesting molecule out in poster-size....
Im wondering how this molecule can have such a high brisance while having an extremely bad oxygen-balance, although the molecule is a big one, and
this may often contributes to high crystal densities, therefor high detonation velocities...
The bad oxygen balance becomes obvious if considering following decomposition equation:
C18H26O2(O2)5 > 12CO + 6C + 13H2
(additional 18,5 moles O2 required for zero oxygen balance)
Im not familiar with the methods of measuring detonation velocities, but so far, DPPP is by far the most interesting energetic material ive heard of,
so i propose to make another brisance test:
If acetone peroxide detonates at around 5000 m/s, and DPPP at 9000M/s, then there has to be a significant difference in the size of shattered
particels of the surrounding material.
For example, if one sticks a blasting device with a high detonation velocity in some kind of soil, the shattered parts of the soil have to be much
smaller, than using one with much lower Vdet.
So it would be very interesting to compare AP and DPPP to see, wether the 9000m/s could be true....
Patents never lie, especially the european ones(american patent office also patents perpetua mobile), but i find it not very promising, that the
patent also says the explosion temperature is 200°C, rather than being 2000°C(maybe a printing error), so im at least careful with this
information....
HLR
P.S: C`m on!-Give this thread the start it deserves....
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
200*C could be the temperature at which it explodes, and not the temperature of the explosion...
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
HLR, formation of H20 is favourable over that of CO.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
vulture)H2O is NOT favoured over CO, the equation has to be right.
Check this:
http://www.fas.org/man/dod-101/navy/docs/fun/part12.htm
H2O is at least favoured over CO2...
HLR
Nick F)Oops, were is my mind these days!! - You are right.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
Damn.......thats not my day.
Heres the right link:
www.fas.org/man/dod-101/navy/docs/fun/part12.htm
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I'm sorry, but that is bogus. If it's right what is being stated there, then TNT would not produce carbon upon detonation. It produces carbon because
firstly the hydrogen is burned to H2O and no more sufficient oxygen is present to oxidize the carbon.
Also, another flaw in there. Metal additions to an explosive get burned as last, simply because they are not part of the molecule.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
Double-damn!!(if you´re right)
well i thought it was a serious site...
do you know a better scientific source?
Gnnnnn, HLR
P.S. has anyone yet synthesized DPPP to compare with AP??-im burning of interest...and i dont have time this week to do it myself.

|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
C6H5N3O6 > CO + C + 5/2H2 + 3/2N2
Carbon is left after decomposition in any case.
So could u please proof what u said, im a little bit confused now..
HLR
P.S. Did u really check that site? - It seems to be a navy-site, and it doesnt look like a "bogus"-site at all.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
ähh, C7H5N3O6>6CO + C + 5/2H2 + 3/2N2
sorry, but i did some severe bad-concentration-errors in the last equation.....please ignore them.
This one is right.
HLR
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Fascinating
This compound does strike me as very interesting. Is anyone here fluent in German? The only parts of the patent that I can understand are the chemical
formulas. If somebody will make a nice translation/summary of this patent I will gladly credit you and add this document to the library!
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
I could translate the most important parts of the patent.
Watch out for the next post.
HLR
|
|
|
kingspaz
Hazard to Self
 
Posts: 55
Registered: 23-7-2002
Location: UK
Member Is Offline
Mood: No Mood
|
|
vulture, nice drawing. i would guess it to be quite stable in relation to AP since it doesn't have a cyclic nature, similar to HMTD.
also i have consulted chemistry of explosives and it says in severly oxygen deficient explosives then hydrogen is oxidised first. i would guess this
to be due to entropy as one mole of O2 can produce 2 moles of H2O as oposed to 1 mole of CO2 (greater product disorder) thus over ruling C's greater
reactivity. but like i siad thats a guess. i can't even remember if C is more reactive.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
Here it is!
German patent DE 1951660.
1969
Diphoronpentaperoxid C18H26O2(O2)5
field of invention: the invention refers to an explosive which has special properties that make it suitable especially for mining and as well for
military purposes.
Task: The invention wants to make blasting operations cheaper and easier.
Solution: Following cheap substances are required for synthesis:
1part conc. hydrochloric acid(HCl)
1part acetone (CH3COCH3)
2parts 30% hydrogen peroxide(H2O2)
Process of manufacture:
1part of acetone are added to 1part hydrochloric acid, through which acetone polymerizes to phoron; then 2parts of hydrogen peroxide are added,
through which two molecules of HCl bond with the two C-double-bonds of the phoron.
In the course of the reaction free chlorine
develops, which bonds with 3 hydrogen atoms of the phoron to give 3 HCl(see drawing) and bonds itself with phoron.
Hydrogen peroxide causes the chlorine atoms to split off the phoron and the peroxide itself bonds with the phoron.
Because of the fact, that one peroxy-bond stays half-opened, one also open phoronperoxide adds to give diphoronpentaperoxide, which precipitates from
the solution in crystalline form.
Properties: a)insoluble in water
b)still explosive when heavily contaminated
c)detonation velocity approx. 9000m/s
d)low detonation temperature approx. 200°C
e)does not develop smoke upon detonation, does not smell, no solid residues.
f)ignition by fire, blasting cap or electric
g)does not decompose at long storage
h)yield of manufacturing process approx. 90%
with best regards, HLR
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Added to library
The patent translation that you supplied has now been added to the sciencemadness library. When there is more information available about this
substance, I will add it to the existing document.
The patent is not very specific about reaction conditions or times. I have just added one volume of HCl (approx. 31%, not concentrated) to one of
acetone. The mixture became cloudy and the flask warmed up a little. I am going to warm this on a hotplate for a while to see if there is any further
sort of reaction visible, then chill it in the freezer to see if any phorone crystallizes out (m.p. 27 C according to chemfinder.com). Then we will
see if I will move to the next stage or not...
One thing that just came to mind: when I was young and foolish, I experimented with acetone peroxide, not really knowing what it was, and so did many
of my friends. One of these friends once claimed that he had made a batch of acetone peroxide using a huge amount of HCl. He claimed that the mixture
grew hot enough to boil, and that when it had calmed down he was left with crystals large enough to be easily visible to the naked eye, and that they
had a slight yellowish cast. He also claimed that, unlike other batches of acetone peroxide, this one would explode, unconfined, even in the smallest
amounts.
So, did he merely have a unique physical modification of acetone peroxide? Did he perhaps form some of this DPPP as well? Or did he make the whole
thing up to amuse me...
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Here's everything I know about phorone (all information from my chemical dictionary).
Formula: (CH3)2CHCOCHC(CH3)2
Density: 0.8791g/cm3 at 20C
Boiling point (760mm Hg): 197.9C
Freezing point: 28.0C
Vapor pressure: 0.38mm Hg at 20C
Properties: Yellow liquid or yellowish green prisms.
I weep at the sight of flaming acetic anhydride.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I just consulted several other references. It seems that the melting point of phorone really is 28C. Phorone is very weakly soluble in water (0.1g
dissolves in 100mL of water at 50C). It is soluble in ether and alcohol.
I weep at the sight of flaming acetic anhydride.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
That's excellent news!
About it being yellowish liquid, that is. Because my HCl/acetone mixture on the hotplate has started to turn a golden yellow.
I didn't realize this until I searched today, but DPPP has recently been discussed a tiny amount on the E&W Forum.
Here's what Microtek had to say:
| Quote: |
Allright, I'm afraid I got quite un-scientifically impatient to find out more about the diphoronepentaperoxide, hereafter referred to DPPP, so after
slowly and carefully reading through the pages, I searched the german patent database and found that the .gifs were copies of those pages. I then made
a synthesis as outlined in the patent: 5 mL acetone was mixed with 5 mL part HCl 30%, heat was evolved and very slight bubbling was observed ( I know
that it is most likely parts by weight, but as I said I was impatient ).
Once the liquid had cooled, 0.5 mL was transferred to a test tube and 0.5 mL H2O2 40% was added. At first drop by drop, then as nothing untowards was
happening, I added the rest.
A white precipitate immediately appeared and the mix was allowed to sit undisturbed for 10 min. After this period the product was filtered off, washed
and dried.
Then I started examining the it and found that it smelled exactly like acetone peroxide, but was a fine amorphous powder like HMTD. Upon ignition from
flame it flashed like HMTD or AP, but if placed on a piece of Al foil above a flame, the powder melted and then detonated violently. Thus far,
everything except morphology pointed at AP, but then I did the impact sensitivity tests. I performed my standard qualitative test which consists of
placing the sample in a piece of Al foil which has been folded to form a small envelope. This envelope is then placed on a steel anvil and a
carpenters hammer is used to pound the sample. It was quite hard to get the sample to detonate; it took blows consistent with what PETN requires, and
when the flat, un-exploded samples still in their envelopes were held into a flame they didn't detonate but only flashed as before.
The final test I did was to pack about .25 gram into a 2 mm pipe attach a fuse, and ignite - no detonation, only flashing which ejected the rest of
the fuse. Further testing will follow. |
Note that he said that the product he obtained smelled and behaved like acetone peroxide. If it looks like a duck and quacks like a duck...
I am also doubtful that he obtained DPPP since phorone does not rapidly form at room temperature (as my current experiment shows). His unusually
impact-insensitive acetone peroxide may actually not be that amazing, since he said that he obtained it as a very fine powder, which is different from
the standard physical form as obtained with lower amounts of acid.
Mmmm, today is a great day for mad science.
|
|
|
Hoffmann-LaRoche
Hazard to Self
 
Posts: 64
Registered: 10-9-2002
Location: mid-europe
Member Is Offline
Mood: No Mood
|
|
Density measurement
most likely, the compound has to have a higher density than AP, as it has such a high detonation velocity, and the molecule is simply much bigger!
So: make again a small batch(are the given mixing proportions by weight, or less likely, by volume ?). ?).
It would be best if somebody took the time to make the stoicheometry check using the reaction equations in the patent therefor.
Then, place some of the -supposed to be- DPPP-crystals in a measuring flask-let´s say a 10ml measuring flask, and exactly 5.0 gram of the DPPP, so
that we can recalculate the density of the DPPP, when the flask is then later filled up with water from a pipet or a buret.
Dont forget stirring.
density(DPPP)=5,0g/(10mL- ml added water from buret) [g/mL] or [g/cc]
HLR
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Here's the correct structure.
If you look at this you'll see that first the HCl serves as a catalyst to "rearrange acetone to phorone. Then hydrogen peroxide frees chlorine from the HCl which
oxidizes the phorone to phoronedihydrogenchloride. After that more HCl converts it to pentachlorphorone which gets oxidized by the oxygen (EN O > EN
Cl) and forms the peroxide bonds where the chlorine was previously bonded.
According to me the hydrogen of the H2O2 will then form HCl with the residual chlorine.
So one could detect succesful reaction by carefully monitoring, because after the phorone will be peroxydized, the pH should drop because of HCl
formation.
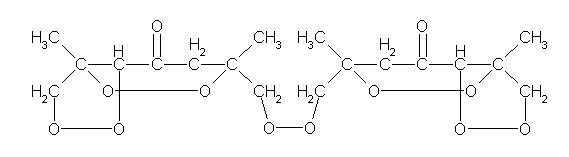
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
| Pages:
1
2
3
..
37 |