| Pages:
1
..
7
8
9
10
11
..
15 |
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Of course I know the danger. For this reason, glue drips only on the edge that is 2.5 cm away from the nearest mixture. The glue only flows 3-5 mm
from the edge to cavity. So 2 cm from the mixture. All of this was tested and measured without active substance. In the case of Lithexal, heating to
ignition temperature is excluded. It is a very insensitive substance. Even a drops of glue, that land directly on a pile of Lithexal will do nothing
at all.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
To diversify the thread, I attach the crystallization process of Cu(ClO4)2 x 6H2O. Procedure: 20g HClO4 68% + 60g dH2O + 20g CuCO3. The solution is
boiled at about 100 Celsius for about an hour. An acidic solution is formed, filtered from exces CuCO3. After filtering slowly evaporation at 60
Celsius. After concentration, the crystals are filtered. Proceeds will be announced later.

Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 442
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
This is a way of making Cu(ClO4)2.
This procedure yields a theoretical 11.6g CuII(ClO4)2 starting from 10g NH4ClO4. Not sure how pure your copper perchlorate needs to be, but this is
pretty pure and easy. I hope it helps.
CuSO4*5H2O + 2NH4(ClO4) + XH2O=> (NH4)2SO4 + Cu(ClO4)2*xH2O
10.62 g CuSO4*5H2O
10.00 g NH4CLO4
50.00 ml dH2O
>100 ml Acetone
Heat dH2O up to boiling
Dissolve all CuSO4
Dissolve in all NH4ClO4
remove heat once all dissolved
Add at least =/> 100 ml Acetone and stir well. if you are above acetone bp, solution will boil when acetone is added and large clumps of sulfate
will be produced instantly.
(NH4)2SO4 precipitates as a white sandy insoluble, it has a layer of copper II ions making it light blue on surface.
Filter off (NH4)2SO4 with copper contamination
Rinse with acetone
light blue filtrate collected contains CuII perchlorate, and should contain no solids at all.
Boil down this acetone/perchlorate solution to evaporate or just leave alone and allow evaporation.
When a white solid begins to appear around meniscus during boiling, remove from boiling and allow to cool.
Blue Cu II Perchlorate crystals will form on cooling.
This procedure can be doubled to 20 g NH4ClO4 & 21.24 g CuSO4*5H2O with only 50 ml of H2O (100ml H2O doubling is not necessary, less water helps
to precipitate out the sulfate on addition of acetone).
      
[Edited on 12-12-2022 by Hey Buddy]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Very easy and interesting , thank Buddy....
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Here are the first results.
Unfortunately, Cu(ClO4) 2 prepared from CuSO4 (and after adding 20% hexamine = called Cu8) shows poor detonation properties. Cu(ClO4)2 x6H2O should
have a melting point of 82 C. This one clearly does not. It does not melt, but strangely enough it burns by itself, without the content of fuel, i.e.
hexamine. The perchlorate prepared in this way contains many impurities, most likely from acetone. Therefore, it is flammable in itself. The
preparation process will require optimization including recrystallization. However, this is a method that could lead to a simple preparation of
neutral Cu(ClO4)2 in the future. Which has not yet been described anywhere. Which is a great benefit. Because HClO4 + CuCO3 produces an acidic version
of crystals that cannot be neutralized with NaOH, KOH, LiOH, NH4OH. Due to impurities, this method of preparing copper perchlorate and hexamine (Cu8)
achieves poor results. Which is evident from the pictures. This Cu8 substance has DDT properties. Which is good news. But it can't fire ETN. Only
causes partial detonation. Small remnants of unexploded ETN were observed in the crater. The figure contains reference values for 0.3 g ETN full
detonation and for 0.3 g Lithex full detonation. In the sense of a highly pressed output segment. Full power Cu8 is in this thread above. And should
by basically same as Lithex. (page 2, shooting into alu brick)

[Edited on 12-12-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 442
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by Laboratory of Liptakov  | Here are the first results.
Unfortunately, Cu(ClO4) 2 prepared from CuSO4 (and after adding 20% hexamine = called Cu8) shows poor detonation properties. Cu(ClO4)2 x6H2O should
have a melting point of 82 C. This one clearly does not. It does not melt, but strangely enough it burns by itself, without the content of fuel, i.e.
hexamine. The perchlorate prepared in this way contains many impurities, most likely from acetone. Therefore, it is flammable in itself.
[Edited on 12-12-2022 by Laboratory of Liptakov] |
Darn. I thought it might be pure enough, sorry. Acetone *should not react, but if it is burning without fuel, there is contamination, it could be
acetone reaction taking place. If an acetone reaction is taking place, it may be possible to reduce the acetone reaction by eliminating heat.
In the provided procedure for Cu(ClO4)2 from sulfate/AP, heat is applied to cause dissolution of the sulfate and perchlorate in a *minimal amount of
water. However, even if no heat is applied, both reagents can still be dissolved in water. Dissolving both reagents will require more water and
kicking out sulfate will require more acetone, but in absence of heat, or alternatively, a lower heat, I believe any reaction with acetone should be
much-less catalyzed, as acetone should be unreactive to the perchlorate I believe.
CuSO4 dissolves at around ~30 g / 100 g H2O and NH4ClO4 dissolves at ~20 g / 100 g H2O at room temp.
The acetone is used to crystallize out the ammonium sulfate which is completely insoluble in acetone. To cause precipitation, the water to acetone
ratio must be wide enough to cause the sulfate to fall out. The acetone is miscible with water, so it can be added to any qty water at a lower
temperature at whatever concentration is necessary which forces sulfate to kick out.
therefore at 25 C, theoretically, 10.62 g CuSO4 and 10 g NH4ClO4 should dissolve minimally in ~85.4 ml dH2O, of course probably round up to 90 ml
dH2O, 180 ml of acetone, probably a bit more, should reject out the sulfate.
Im not saying that this would produce a more suitable Cu(ClO4)2 for sure, but if contamination by acetone reaction is actually happening, this should
minimize reaction contamination.
Just maybe that would cause a crystallization of a more-pure Cu(ClO4)2 that could be suitable and expedient.
[Edited on 12-12-2022 by Hey Buddy]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Thanks Buddy. I will return to CuP synthesis sometime later. The main direction of research is Lithex. When Lithex is dried and anhydrous, its DDT
reliability is 100%. Moreover, his brisance is basically the same as ETN. Another thing, the ratios between LiClO4 (anhydrous) and hexamine may not
be exact. The range is 75 - 85% LiP / 25 - 15 hexamine. And it always works. Of course, a stoichiometrically balanced mixture is best. Oxygen balance
LiP OB = + 60.15. Hexamine OB = - 205.41. = - 0.929 balance on CO2. LiP can be trihydrate, dihydrate, monohydrate and anhydride. Always dry LiP at 175
Celsius for best results. Then weigh, for example, 1.54g of LiP (anhydrous) and add 0.46g of hexamine. (anhydrous) Then create a liquid by adding dH2O
and evaporate again at 175 C. The temperature of the Lithex and the filling utensil must not drop below 40 C. Against moisturity. After sealing in a
hermetic cavity cn be temperatury any.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
During further tests, Lithex is shown to detonate consistently and reliably. The dosage was only 900 mg. Therefore, the crater is not as deep as from
1g ETN. Also, the density of Lithex was slightly lower, only 1.7 g/cc. A 3% addition of aluminum Alu - bright was used. However, the diameter of the
crater is the same. This means that Lithex can fully replace ETN in many applications. His DDT is reliable and his brizance is very similar.

Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
yobbo II
National Hazard
   
Posts: 769
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Will Calcium perchlorate (easy to get) and copper sulphate not give pure copper perk.
Calcium sulphate in quite insoluble.
Yob
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
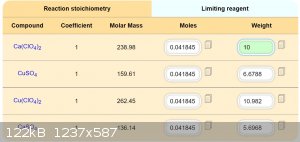
If is Ca(ClO4)2 easy available, is it maybe best way for preparation Cu(ClO4)2. Good idea, thanks.
Interesting fact: Lithium perchlorate with hexamine (Lithex) has a special property after drying at 175 Celsius. Its structure and behavior is very
similar to talc. A very fine powder is always formed which is partly slippery. Like crushed the talc.
[Edited on 27-1-2023 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by Laboratory of Liptakov  |
As already indicated, at least 2 crystalline barriers must be overcome when drying LiP. At 80 and 120 C. The melting point of completely dry LiP is
236 C. Boiling point - decomposition 400 C. For this reason, there is no need to worry about decomposition during drying to the anhydride. Caution, do
not use Teflon for drying. LiP immediately reacts with Teflon at 80 ° C and begins to blacken. Even the stainless steel surface is not perfect. When
LiP is melted, there is a slight browning of pure factory LiP. The same effect is observed when the Li8 mixture is dried. After drying, the mixture is
slightly gray. However, its energy properties will not affect it for the worse.
[Edited on 24-1-2022 by Laboratory of Liptakov] |
I made some lithium perchlorate over the weekend as a way to make use of some partially degraded lithium metal (thanks j_sum1!). I thought I would try
to use a small portion of it to make some Li8. I had not read the above quoted post from earlier in this thread and used a teflon coated pan. I added
the lithium perchlorate trihydrate and hexamine to a pan and started to apply heat. The progression from trihydrate to dihydrate progressed fine, but
when the temperature hit about 120 C on the surface of the pan the liquid started to turn yellow and then brown. As the material darkened from yellow
to brown the hexamine seemed to begin to decompose, as evidenced by an ammonia and fish odour. At this point it was removed from the heat and
solidified into a brown crumbly solid. Given the decomposition I did not try to proceed to the anhydride. I think what I ended up with was the
monohydrate, it performs similarly to the Dr's observations for the monohydrate.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
It's a waste of valuable LiClO4. And an error in the manufacturing process. Lithex reacts with teflon always. It was try and confirmed. The stainless
steel pan is proven and tested. With a straight flat bottom. And a stainless steel mixing plate. Just like the pictures in this thread. (and video)
Even small deviations from the production procedure usually end in failure. This is a generally known rule, especially in chemistry. Recommendation:
1) dry 2g of LiP trihydrate to anhydride. Observe its behavior and repeated dissolution in a stainless steel pan. It must remain white. (this is a
test for impurities)
2) keep the temperature of the stainless steel surface at 170 C. Stir for 1 minute at this temperature.
3) put the hot dry LiP into an airtight jar and seal immediately. (if it's white or just slightly yellow = OK)
4) do the same test for impurities with 1 g of hexamine at 120 C. If it remains white or only slightly yellow, (OK) seal in an airtight jar.
5) Wash the pan.
6) Pour 15g of dH2O onto a dry, clean pan and pour 0.46g of hexamine into it. Which was weighed on aluminum foil.
7) Weighing 1.54g of LiP anhydride on aluminum foil. And pour into the water. (exact ratio 77%LiP anhyd. + 23% hex)
8) increase the temperature to 120 C and keep stirring. A dry white powder should form. (slight yellow = OK)
9) Increase the temperature to 170 C. (160 - 170) The powder should dissolve again. Keep stirring and rubbing the powder with a stainless steel plate.
A talc-like consistency will form. This is dry Lithex. Smell of hexamine is normal sublimation at this temp.
10) immediately seal hot Lithex in an airtight jar.
11) do the burning test and hammer test according to the video (from 3:47)
https://www.youtube.com/watch?v=BubkrGVEwF8&t=27s
12) add 0.05g Alu powder and shortly shaking. Increase DDT effect.
[Edited on 30-1-2023 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
underground
National Hazard
   
Posts: 714
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
May i ask why you have to use TACP or LL8 Or Cu8 or LITHEX or whatever when ETN can replace all of those ? ETN is easier to prepare not hygroscopic
and not sensitive yet high in performance. Nitrating erythritol with drain cleaner and any kind of nitrate salt is much more easier than preparing
perchlorates.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Because ETN has usually difficult DDT itself. TACP, LL8, Cu8, Lithex has easy DDT. TACP and CHP are not hygroscopic.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
underground
National Hazard
   
Posts: 714
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Are not all perchlorate salts need a metal cavity to go DDT, just like ETN ? Are any of these capable to DDT into a plastic straw like other
primaries, for example HMTD ?
|
|
|
Microtek
National Hazard
   
Posts: 919
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I question the statement that ETN is not sensitive.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by underground  | | Are not all perchlorate salts need a metal cavity to go DDT, just like ETN ? Are any of these capable to DDT into a plastic straw like other
primaries, for example HMTD ? |
Nickel bis-aminoguanidine diperchlorate does not need any confinement, same with the copper equivalent. I am sure there would be others.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
The easiest of the way of acids is ETN and SA-DS in plast confinement. The easiest of perchlorate way, is the Lithex and metal confinement...
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Damn straight.
ETN is right up there with nitro mannitol for impact sensitivity.
It may not make DDT in smaller amounts too easily at room temperature but in melted state it is about as shock sensitive as dry liquid nitroglycerin
at room temperature.
People have washed it of residual acids by agitating in hot water while melted rather than dissolving in solvents & recrystallizing, some have had
notable accidents.
Some have melt cast it to increase density, accidents have occurred there too.
Whole threads have been written on provoking it to behave like a flame sensitive primary towards ignition, it's far from impossible.
Powerful, easy to make, stable and safe to handle. Pick any 2. (OK, phlegmetized PETN is almost 3 out of 3.)
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|

Due to next research was developed an interest substance. ( working title Brightelite) Which is not full power material for use as initiation for
secondary material. But his properties are interest. S a base of mixture is formula: AP 80 + CuO 4 hexamine 8 in part by weight. (No. 4). This
substance is of course weak, with very positive oxygen ballance, but any way, brisantion is there. Lab. preparation is 20g dH2O on stainless
surface, + 4g AP + 0.2g CuO + 0.4g hexamine. Boiling solution, evaporate and gringing on very fine powder. Operation without accident at 80 C on
surface friction. Second step is adding nitrocellulose commerce grade 12.4 N 0.35g + Aluminium bright powder 0.35g. + 2g Acetone for dilluting of NC.
This mixture has OB + 2.0 on CO2. Mixing on porridge at 20C, evaporate and grain 1x1 mm. And is more power. (No. 1 +2) . There is enough power for
full initiation of ETN now. (No.3). For last test (No.5) was used nitrocellulose with over 13% N. Resluts show clear bigger brisantion. Mixture
(grain) is pH neutral, no-hygroscopic with good manipulation properties. For all test was used output segment 300 mg, ETN density 1.65. Brightelite
density 1.5 g /cm3. As itself is weak, but can provide easy way for (maybe even reliable) of initiation ETN in solid metal cavity. Friction and
impact test show on almost same properties as CHP. Also his burning speed is similar with similar color of flame.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Well, THAT is quite snappy for a mixture of common pyrotechnic materials. Have you tried zapping it with a piezoelectric spark yet?
Could you describe containment, ignition used? What does it do if ignited in the open...
I would expect substitution of a commercial high nitroglycerin smokeless propellant such as "Bullseye" for the higher nitration nitrocellulose of test
5 might yield similar or even greater brissance.
L

[Edited on 2-12-2023 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Reading your recent research I'm so glad I didn try those pyrotechnic compositions I was thinking about a few years ago...
I was going for ammonium perchlorate, hexamine and CuOCl the first two being quite "neutral" in terms of color production.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Herr Haber  | Reading your recent research I'm so glad I didn try those pyrotechnic compositions I was thinking about a few years ago...
I was going for ammonium perchlorate, hexamine and CuOCl the first two being quite "neutral" in terms of color production.
|
The ammonium perchlorate + copper benzoate "new blue" star mix with nitrocellulose binding was quite good, I suspect I might have had "issues" if I'd
tried to increase the light output with fine metal powders...
Hexamine itself has a reputation as cool burning fuel which generally slows down star composition burn speeds when used with POTASSIUM perchlorate. I
wonder what the mechanism of the speeding up to primary like behavior here is? What species might be produced by any reactions during that initial
water mixing + heating to dryness? Copper hexamine perchlorate? Did the heating and drying produce heavy ammonia odors?
[Edited on 2-12-2023 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Bert: .......
On page 8 this thread ...posted on 16-9-2022 at 20:38.....is photos of system electric ignition and filling procedure assemble. Sensitivity on
piezoelectric electric spark not tested. (but results can be early)
Confinement in 5 layer in alu foil - candle heating - not tested (but results can be early) Free heap on air burn similar as CHP or BP. (on last
pic.)
Shotgun commerce powder (with NG from pic) was not tested (but results can be early)
Behavior during wet process not show any reaction between compounds. Hot water before evaporate is pure transparent without blue feets.
If is during wet process in water one drop HClO4, (0.05g) instantly arise Cu(ClO4) blue water. But mixture is after hygroscopic and more brizantion.
One drop of NH4OH causes formation TACP, repectively CHP. (no hygroscopic)
Herr Haber,.......
For pyro composition is possible increase CuO on 10% against AP for example. Any tuning on good blue require absence NC (yellow feet) and more tests.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1445
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
So Bert's assumption was correct. Composition: 80 AP + Hex 8 + CuO 4 + Al 7 + Shotgun powder 8 parts..(No. 6) ..show basically same brizantion as
composition with homemade nitrocellulose 7 parts of weight. (No.5) Diameter is only slightly less. Precision repeatedly measurement are No.5 2.7 +
12.5 mm. And for shotgun powder 2.7 x 12.2 mm. Exact results of course require minimal 3+3 tests for both formula, but for orientation results is it
enough. Both formulas has sufficient brizantion for initiation ETN. next:
¨
Test on confinement in alu foil 5 layer , candle heated causes only puffy and rocket effect.
Test on electric spark was without ignition of Brightelite. (directly pour 0.1g into the lighter hole, 10x sparks)
Burning Brightelite with shotgun powder has slightly slower burning on free heap. Than with homemade NC.

Now I remembered: the homemade nitrocellulose used was prepared by double nitration. Commercial fibrous NC was re-nitrated. The PLM method was used
for its cleaning and stabilization....
https://www.youtube.com/watch?v=oVZkPWxj5WE
Thus 4 years old, but good stabilized NC with nitrogen content maybe over 13.5 %.
[Edited on 13-2-2023 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
| Pages:
1
..
7
8
9
10
11
..
15 |