| Pages:
1
2
3
..
15 |
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
LL8 from Dr. Liptakov
Due to the unexpected properties of the examinate substance, I would like to start a new thread on his theme. And also because almost nothing but safe
and comfortable initiation is addressed in energetic materials.The substance was gived working name LL8. Because CHP is closest to the new
substance, it is used for comparison. Just like the notorious ETN.
Basic results are on two pictures. Output segment with high density was set on 400 mg. Starting DDT segment with low density set on 250 mg. Other
space is filled classic BP to full height.
Density for output segments ETN in steel cavity 6/8 x 50 mm. = 1.75g /cm3 + - 0,03 g/cm3.
Density for output segment LL8 in steel cavity 6/8 x 50 mm. = 1.87g /cm3 + - 0,03 g/ cm3.
Density for output segment CHP in steel cavity 6/8 x 50 mm. = 1,56 g /cm3 + - 0,03 g/ cm3.
Density of LL8 / 250g which is use a like primary for all tests 1,4 - 1,6 g/cm3.
BP is pressed on cca 20 - 30 Kg.


Control measurement on pic.7,8 with exact output edges.


Differences between LL8 / CHP
1) LL8 is demonstrably more brizantely than CHP.
2) LL8 not releasing NH3 gas from molecule, is without smell.
3) LL8 can be mixed with metals and other EMs with less doubt.
4) LL8 has preparation procedure without ammonia water.
Properties which are same or similar for LL8 and CHP:
5) No hygroscopicity as free heap on air within days at laboratory huminidity.
6) Primarily - secondary energetic material.
7) Detonator must have metal cavity. (Soft cavities are in exam)
8) Friction sensitivity similar (exact values later, subjectively without accident)
9) Impact sensitivity similar (exact values later)
10) Burning on air only, without DDT, with speed similarly as quality BP
11) Not sensitive for over-pressing dead
Disadvantage LL8 against CHP:
1) Basic precursor HClO4 is usually expensive or unavailable. (Preparation HClO4 from NH4ClO4 available on LL pages YouTube)
2) Preparation Cu(ClO4)2 can be difficulty against preparation TACP.
3) Other method preparation Cu(ClO4)2 from else perchlorates salts not found. Experienced chemists can complement it.
Reagents for preparation:
Cu(ClO4)2 (hexahydrate)........2,4g
Hexamine ...............................0,6g
Ethanol commerce grade .......1,5g
H2O distiled.............................0,5g
Preparation:
Mixing CuP and hexamine on inert surface at 20 C with Ethanol and distil water.

Make the fine slurry. Pour slurry on inert surface 70 - 80 C. Evaporate EtOH+H2O and make the grain using a 1x1 mm sieve. Notice: During evaporate
arises gas which irritate eyes and nose, same effect a like at second glue. Ventilation recommended.
Drying 20 minute on inert surface at 50 - 80 C.

Final color after drying on picture:

Cu(ClO4)2 can be prepared, Method 1:
Heate solution CuO + HClO4 of 40% conc. with 2O% exces CuO for 80 C. Wait on pH 4. Neutralize by drop of ammonia water for pH near neutral. Cooling
on zero C. Can arised small amount NH4ClO4 which crystallize firstly. Separate exces CuO+NH4ClO4 on filter. Evaporate (blue transparent) solution at
100 C on inert plate. (Tested,works in det.)
Method 2:
Heate solution of HClO4 (30 - 40% conc.) at 20 C and add CuCO3 in small portions. Arises CO2. Add exces 20% CuCO3 until to zero bubble CO2. At 20C.
Heate on 80C and wait on pH 4. Neutralize by drop of ammonia water for pH near neutral. Cooling on zero C. Arise small amount NH4ClO4 which
crystallized firstly. Separe exces CuCO3+NH4ClO4 on paper filter. Evaporate water from solution at 100 C on inert plate.(prepared,works in det.)
Solubility NH4CLO4 11,5g in 100g H2O at zero C.
Solubility Cu(ClO4)2 . 6H2O...146g in 100g H2O at 30C, melting point 82 C as hexahydrate.
Tested also in different ratios, results on witness plate: (without picture)
CuP : HEX
50 : 50 = failed, flat steel
70 : 30 = failed, slight bending of steel only
80 : 20 = best results, bigest hole
85 : 15 = good results, but smaller hole
Test for stability LL8 in detonator. One detonator filled before 14 days and storage. Second detonator filled after 14 days, from else batch, but with
two identical design and conditions. Result on pic9.

Detail preparation LL8 and basic properties published from today without the right to a reward or patenting. For all researchers of science madness
free.
Dr. Liptakov.......
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
karolus28
Hazard to Self
 
Posts: 51
Registered: 14-4-2019
Location: EU's Brazil
Member Is Offline
Mood: zgrzyt
|
|
mysteroius bhoice makes copper chlorate by membrane electrolysis, maybe would work using sodium perchlorate instead of chlorate? https://www.youtube.com/watch?v=Fdst_kxZL2Y
Hi, please read about exif data.
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Very Cool!!
Why cannot potassium perchlorate be used? What is special about copper perchlorate? Why not ammonium perchlorate?
What about ammonium perchlorate and guanidine or creatine nitrate?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
The Cu (ClO4) 2 used was found to still contain the HClO4 from which it was made. The residual acid, together with Cu (ClO4) 2 together with hexamine,
to form hexamine copper diperchlorate + another unknown substance. The residual acid is evaporated in a pan at 80 DEG C. and the mixture becomes
non-hygroscopic in this step. Therefore, a pungent gas is smelled during evaporation. I estimate that the whole process will not be possible without
HClO4.
Of course, it is possible to mix everything with everything and look for another 10 different similar mixtures. However, in such a case, the work (for
free) will take an infinite amount of time. The above substance works. And it has unexpectedly good properties as described.
The development of similar, similarly powerful energy substances costs millions in commercial laboratories. And after, all the information about them
will end up in the military government vault.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
specialactivitieSK
Hazard to Self
 
Posts: 94
Registered: 21-10-2014
Member Is Offline
Mood: No Mood
|
|
How about other metals like lithium or Selenium?
Can be used NH4ClO4 and Not HClO4 in some reaction ?
[Edited on 9-1-2022 by specialactivitieSK]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
You can try everything possible. You may get even better results. I'm not a chemist. I am only an inventor with demonstrably clear results in the
field of energetic materials.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | The Cu (ClO4) 2 used was found to still contain the HClO4 from which it was made. The residual acid, together with Cu (ClO4) 2 together with hexamine,
to form hexamine copper diperchlorate + another unknown substance. The residual acid is evaporated in a pan at 80 DEG C. and the mixture becomes
non-hygroscopic in this step. Therefore, a pungent gas is smelled during evaporation. I estimate that the whole process will not be possible without
HClO4.
Of course, it is possible to mix everything with everything and look for another 10 different similar mixtures. However, in such a case, the work (for
free) will take an infinite amount of time. The above substance works. And it has unexpectedly good properties as described.
The development of similar, similarly powerful energy substances costs millions in commercial laboratories. And after, all the information about them
will end up in the military government vault. |
I hate doing this because it seems like I am minimizing your work! I am trying to take out the hazardous and excotic chemicals such as perchloric
acid. What you discovered is amazing! I do wonder. What if you mixed ammonium perchlorate, copper ammonia, hexamine and water together.
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 191
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MineMan  | Quote: Originally posted by Laboratory of Liptakov  | The Cu (ClO4) 2 used was found to still contain the HClO4 from which it was made. The residual acid, together with Cu (ClO4) 2 together with hexamine,
to form hexamine copper diperchlorate + another unknown substance. The residual acid is evaporated in a pan at 80 DEG C. and the mixture becomes
non-hygroscopic in this step. Therefore, a pungent gas is smelled during evaporation. I estimate that the whole process will not be possible without
HClO4.
Of course, it is possible to mix everything with everything and look for another 10 different similar mixtures. However, in such a case, the work (for
free) will take an infinite amount of time. The above substance works. And it has unexpectedly good properties as described.
The development of similar, similarly powerful energy substances costs millions in commercial laboratories. And after, all the information about them
will end up in the military government vault. |
I hate doing this because it seems like I am minimizing your work! I am trying to take out the hazardous and excotic chemicals such as perchloric
acid. What you discovered is amazing! I do wonder. What if you mixed ammonium perchlorate, copper ammonia, hexamine and water together.
|
First off, let me say that this seems like a very cool invention. I have recently made some TACP by LL's method and I will try this out when I can.
Also, MineMan, you can make perchloric acid pretty easily if you have ammonium perchlorate. It is a method I have done myself, all it requires is some
hydrochloric acid, nitric acid, ammonium perchlorate and a flask. No distillation necessary, though you could always distill from a perchlorate salt
and sulfuric acid. Also, if you have sodium perchlorate, it is considerably more soluble than sodium chloride, so it is possible to make a saturated
sodium perchlorate solution and then add HCl. NaCl will precipitate out and then you can filter off your perchloric acid, and perhaps put it in the
freezer to remove remaining NaCl.
Anyway, the procedure for the method using ammonium perchlorate without distillation is here:
Quote:
https://prepchem.com/synthesis-of-perchloric-acid/
50 grams of ammonium perchlorate are added to 60 ml of distilled water in a 500 ml beaker. 40 ml by volume of 68% nitric acid are added to the
ammonium perchlorate solution and the solution is heated to a rolling boil (88-92°C). While heating, prepare a solution 15 ml of 30% hydrochloric
acid in 25 ml distilled water. Once the ammonium perchlorate nitric acid solution is above 80° C, begin adding dropwise, the hydrochloric acid
solution over a period of 25-30 minutes. The rate of addition should be sufficient to replace the lost water from boiling to ensure the volume
remains constant. Once all the hydrochloric acid is added, allow the solution to boil for 30 minutes before increasing the heat to 135-140° C to
drive off the remaining water, nitric and hydrochloric acids. The resultant solution should be pure perchloric acid. If there is any ammonium
perchlorate remaining, it will crystalize out upon cooling.
-------------------------------------------------------
I have used this method and it does work, it's pretty easy and can give perchloric acid that is certainly concentrated enough for making salts.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Elemental Phosphorus: Thanks .... When I saw the first holes from this substance,
I couldn't believe my eyes. Some were as large as those of ETN. Some even bigger. With sharp bottom edges. More accurate measurements have shown that
it is not as strong as ETN. The current estimate is approximately 90-95% ETN brisance. During further tests, it was found that the presence of ethanol
was not a condition. Just 2g of distilled water is needed to dissolve. Precipitation of fine powder occurs immediately after mixing CuP and hexamine.
Water-insoluble turquoise particles. However, the process also requires heating in a thin layer on stainless steel. Until completely dry. Or another
inert surface. It often happened that the porridge baked in the pan. So I used a steel scraper. At least 30 times. The surface was heated to 80 C at
that moment. And yet nothing happened. Shield, of course, thick gloves, earplugs. And always removed from the area a small amount. And closed aside.
And then the scratching continued. Still no incident.
And also thanks for the procedure for HClO4. It looks easy and is mainly described in detail.
The observation of filter paper burning led to the preparation of a new substance. Which burned so energetically that he blew out any lighter flame.
It was as if a stream of gas with a pressure of several atmospheres was coming out of the paper. Such behavior has not been observed with any other
perchlorate. Sharp blue flame in one direction. Like an autogenous flame. So if the paper burns like this, what will hexamine do?
MineMan...Perchloric acid not smell, not fuming. Is it a like water. From my experience is it most friendly acid between others....
[Edited on 9-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Elemental Phosphorus: Thanks .... When I saw the first holes from this substance,
I couldn't believe my eyes. Some were as large as those of ETN. Some even bigger. With sharp bottom edges. More accurate measurements have shown that
it is not as strong as ETN. The current estimate is approximately 90-95% ETN brisance. During further tests, it was found that the presence of ethanol
was not a condition. Just 2g of distilled water is needed to dissolve. Precipitation of fine powder occurs immediately after mixing CuP and hexamine.
Water-insoluble turquoise particles. However, the process also requires heating in a thin layer on stainless steel. Until completely dry. Or another
inert surface. It often happened that the porridge baked in the pan. So I used a steel scraper. At least 30 times. The surface was heated to 80 C at
that moment. And yet nothing happened. Shield, of course, thick gloves, earplugs. And always removed from the area a small amount. And closed aside.
And then the scratching continued. Still no incident.
And also thanks for the procedure for HClO4. It looks easy and is mainly described in detail.
The observation of filter paper burning led to the preparation of a new substance. Which burned so energetically that he blew out any lighter flame.
It was as if a stream of gas with a pressure of several atmospheres was coming out of the paper. Such behavior has not been observed with any other
perchlorate. Sharp blue flame in one direction. Like an autogenous flame. So if the paper burns like this, what will hexamine do?
MineMan...Perchloric acid not smell, not fuming. Is it a like water. From my experience is it most friendly acid between others....
What percentage is needed to add a perchlorate ion to hexamine, melamine, creatine and other materials. Perchlorate seem perfect for DDT. For
aminoguanidine you can do meta thesis, tho I don’t know why. Does anyone have a discord name who could explain these things further?
[Edited on 9-1-2022 by Laboratory of Liptakov] |
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I see MANY uses for this mixture. One being an excellent catylist for rocket fuel!! Just a few percent
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
In addition, copper perchlorate is fun. I mentioned burning filter paper. Of course, the test paper filter is very hygroscopic. It contains XX
percent Cu (ClO4) 2. 6H2O. Which in itself hygroscopic pretty enough. But it stays dry for 1 minute. When you place such paper on an anvil and strike
it with a hammer, it behaves the same as paper with a drop of nitroglycerin. Attention! Small pieces of paper act like micrometeorites. They get stuck
under the skin. If you don't have gloves. ETN heated on foil has the same effect, thus flying micrometeorites. According to ongoing research, Cu
(ClO4) 2 appears to form high-performance energy mixtures with almost any fuel. Solid or liquid. CuP is very high soluble in ethanol, good soluble in
acetone, insoluble in nitromethane....
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
LL8 impact test
I have one more funny story from the shoot. Impact sensitivity has only been tested once. I assumed that the gases would escape down both sides around
the steel brick. The same amount as in the picture was used. The guide tube will have to be made of something stronger ....

LL8 with the addition of 4% Aluminum bright gives holes with an identical diameter as ETN. And plus, LL8 has more precise and sharper edges on the
output side than ETN. Thats mark of high detonatiom pressure for LL8.

[Edited on 10-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Brightthermite
Hazard to Others
  
Posts: 133
Registered: 26-6-2019
Member Is Offline
|
|
So you suspect that some percent of acid left in the copper perchlorate is necessary. I wonder how much exactly and I wonder if this LL8 can be made
with reagent grade copper perchlorate, then we would know if the acid in necessary.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Compared to distilled water, the pH paper shows a slight acidity. But it is not the acid content that would cause a significant change in the
properties of the final product. This is my current assumption. Furthermore, I do not know how to produce absolutely neutral Cu (ClO4) 2.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
More brisant than CHP and NHN? Wow! I should try it sometime. I'm going to save this for a later experiment.
I know you mentioned how friction and impact insensitive CHP is, but how impact and friction sensitive is LL8? Similar? More or less? As you know my
quest to make NHN was largely because of not only how powerful it is, but also how stable and safe it to handle, load, and press.
Edit: Is the Cu(ClO4)2 (hexahydrate) the same as Tetraamine Cooper Perchlorate? Or is it something different?
Edit2: Will you make a video showing how you synthesize it? Your videos are remarkably fun to watch.
[Edited on 12-1-2022 by ManyInterests]
[Edited on 12-1-2022 by ManyInterests]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
LL8
According tests above is LL8 really brizantely than CHP. NHN was not tested at same conditions. The assumption is that NHN will have a lower brisance
than LL8.
LL8 is very insensitive to friction. Withstands scraping from a hot pan (50 - 80 C) with a steel plate. 30 batches scratched without incident.
On impact is sensitive similarly a like heads pieces from safety matches.
Cu(ClO4)2 is Copper perchlorate. Tetraamine copper perchlorate is (TACP) : [Cu(NH3)4](ClO4)2. From TACP during decopmose is impossible prepare
Cu(ClO4)2
From TACP + HCLO4 is impossible prepare Cu(ClO4)2.
Is necessary classic method HClO4 + CuCO3 = Cu(ClO4)2 + H2O + CO2.
Videos: My information is too accurate. In the case of LL8, almost strategic. If I make a video with a detailed procedure, (and properties) I will
have problems with the authorities.
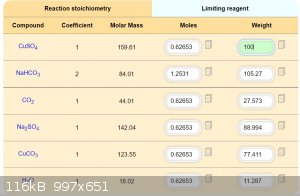
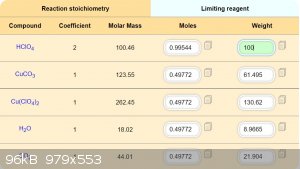
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by Laboratory of Liptakov  | According tests above is LL8 really brizantely than CHP. NHN was not tested at same conditions. The assumption is that NHN will have a lower brisance
than LL8.
LL8 is very insensitive to friction. Withstands scraping from a hot pan (50 - 80 C) with a steel plate. 30 batches scratched without incident.
On impact is sensitive similarly a like heads pieces from safety matches.
Cu(ClO4)2 is Copper perchlorate. Tetraamine copper perchlorate is (TACP) : [Cu(NH3)4](ClO4)2. From TACP during decopmose is impossible prepare
Cu(ClO4)2
From TACP + HCLO4 is impossible prepare Cu(ClO4)2.
Is necessary classic method HClO4 + CuCO3 = Cu(ClO4)2 + H2O + CO2.
Videos: My information is too accurate. In the case of LL8, almost strategic. If I make a video with a detailed procedure, (and properties) I will
have problems with the authorities.
|
I'm having a hard time understanding your writing.
From TACP + HCLO4 is it impossible to prepare Cu(CIO4)2 or do you mean it is possible?
When I was asking for a video. I wasn't asking for an LL8 video, I think I can do it without watching a video of someone else doing it, but I was just
curious about the regular copper perchlorate. I've seen (and downloaded) your video on CHP, it was as clear as it could be. I can figure it would look
similar. However if all you need to make copper perchlorate is perchloric acid and ammonium perchlorate I think I can manage. I have no experience
with making ammonium perchlorate, so this will be my biggest challenge in the future. The only chlorate I have experience with is potassium chlorate.
I have an MMO anode and a titanium cathode. Based on your videos. I will need to invest in a cup type crucible so I can perpare sodium perchlorate
from sodium chlorate (which I never attempted previously).
Also when you mentioned impact sensitivity, you said it was similar like a matchhead? Do you mean safety matches? Those cannot be set off by impact.
Or are you referring to strike anywhere matches with a white phosphorous head?
But I have seen your videos on CHP. If it is tougher than CHP in terms of friction and impact sensitivity then I assume it is very stable and very
safe to handle, load, press, and store. Even more safe than NHN, which is saying a lot.
How flame sensitive is it?
[Edited on 12-1-2022 by ManyInterests]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
LL8
//From TACP + HCLO4 is it impossible to prepare Cu(CIO4)2 or do you mean it is possible?// Is it Impossible.
For prepare HClO4 you need NH4ClO4, HNO3, HCl. Is it method from Horace C. Adams 1925. Described here above.
You can try dry matchhead at 80 C during 30 minute. On hotpan. And after you can use hammer agains the vise. Or similary. And you see the sensitivity
on impact. Without any phosphorus.
NHN is sure more stable than CHP. NHN is good material. But is it work with hydrazine. From this reason I leaved the preparation of NHN.
On flame is CHP and LL8 same sensitive a like NHN.
For exact measurement you need own falling hammer. Is it basic equipment of researcher....
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
OK that makes sense. I'll definitely try that experiment with matches to see how sensitive it is. As long as it is safe to load and press gently with
little danger of an accidental detonation, I think it will be good to work with and I definitely try it.
If it is as powerful as you say then I would think that it is a near perfect primary, and probably may not even need a booster and would work on its
own.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Yes, you understand that correctly. No booster is needed. LL8 is not just a primary compound. It is primary - secondary filling consisting of only one
substance. In a cavity, a single substance (eg LL8 or CHP) differs only in density.
1) With a low density (approx. 1.0 - 1.4 g / cm3) it plays the role of a primary substance. A resistance bridge is placed in this density. This is
where the transition from combustion to detonation (DDT) occurs at a lower rate. For example zero up to 3000m / s.
However, this detonation rate is sufficient to initiate the detonation of the output segment. In which DDT would be (due to its high density)
difficult or impossible or unreliable.
2) The output segment is of the same composition, but with a higher density (1.7 - 1.9) and therefore has a higher detonation rate = pressure. The
outlet pressure is so strong that it can initiate EM based on AN. This is true for both CHP and LL8 substances.
NHN has a detonation pressure 20,8 GPa
CHP has a detonation pressure 22 - 25 GPa.
LL8 has a detonation pressure 27 - 29 GPa.
ETN has a detonation pressure 30 GPa
Another advantage: If there is only one substance, it cannot eat each other.
[Edited on 13-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Bravo LL. Bravo! This is huge! Your focus on perchlorate has paid off. I urge you to try the aminoguanidine perchlorates! They are a tad less
sensitive then CHP, but ddt at 20mg any density. In fact. One detonator was placed next to another. It bent the tube but it not set it off. Again. Not
as safe as CHP, but impossible to set off steel on steel friction.
Bravo LL Bravo
What makes copper perchlorate so powerful, say compared to lithium perchlorate?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Thanks for offer of aminoguanidine. But I am lazy prepare AG, especially, when hexamine (+CuP) give very good pressure. Almost same as ETN.
Any way, interesting reccomendation is it for future.
Yes, Lithium Perchlorate will tested soon.
In my opinion, Cu (ClO4) 2 is an unexplored substance. Respectively as oxidizer with various fuels. Copper holds the molecule together only very
weakly, compared to other metals. Copper provide weak hold in a lot elses molecules. For example Copper acetylide. Just a guess, nothing more.
Thanks for the recognition, MineMan ...
[Edited on 13-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Amazing work as always LL!
I assume your witness plate is mild steel? What is the thickness of the metal?
As others have suggested it would be interesting to try other metals, for example a nickel version of LL8.
I also agree with MineMan that copper or nickel aminoguanidine perchlorate would be interesting.
As you say though, perchlorate is generally more accessible to the amateur than aminoguanidine.
Thanks so much for sharing the results of your work!
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
How would a finely divided mixture of copper hydroxide and ~hexamine perchlorate or hexamine and AP perform?
Not entirely irrelevant:
https://doi.org//10.1615/IntJEnergeticMaterialsChemProp.2011...
https://doi.org/10.1021/jp077278z
|
|
|
| Pages:
1
2
3
..
15 |