| Pages:
1
2
3
4 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Len1, now that I caught myself in reading your posts similarly uncarefully as you do, I reread and realized where all this misunderstanding probably
comes from. I noticed that you seem unaware of the mechanism of these decarboxylations. There are numerous mechanisms by which benzoic and other
aromatic acids can decarboxylate. For example just to mention a few of the more common yielding the Ar-H from Ar-COOH:
a.) p- and o-hydroxybenzoic acids can decarboxylate trough a retro-Kolbe-Schmitt mechanism (inapplicable to nicotinic acid);
b.) Cu powder in refluxing quinoline can be used to promote the radical decarboxylation mechanism (often used in the lab but
irrational to use for something as simple as pyridine);
c.) the thermolysis of alkali carboxylates yielding the decaboxylated and carboxylate disproportionation products (not particularly
effective since the reaction is driven by carboxylate group disproportionation - hence unable to give good yields due to part of the compound forming
dicarboxylic acids);
d.) and finally the decarboxylation in the NaOH melt that I was trying to promote as a better alternative in reply to Leu's post.
Then there are also the decaboxylative ketonization mentioned in previous posts, the oxidative decarboxylations like the Hunsdiecker reaction, the Kolbe electrochemical radical decarboxylation, and many others of little relevance here… and there are also more types for non-aromatic
carboxylic acid.
I was talking about d, a well known reaction mentioned several times on this forum (there is even the experimental by Axt in prepublication). Here is a scheme with the mechanism included just in case I was misunderstood again (I was proposing just to substitute
benzoic acid with nicotinic, nothing more complex than that!):
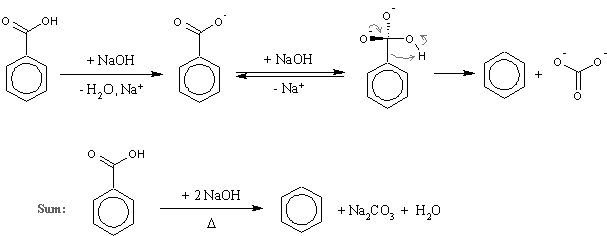
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
You are a tricky fellow. As soon as one condenses what you are saying to a testable prediction, you try to extricate yourself by moving the goal
posts.
If there is any practical meaning to you posts regarding CaCO3 is that its as good a base as Ca(OH)2 - thats what you wrote. But you think it cant be
used for decarboxylatin for another reason - calcium salts produce ketones. Well then, sodium salts you think dont, and it follows sodium carbonate
is as good as the hydroxide.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Huh, finally you read what I wrote correctly!
With a minor exception:
| Quote: | Originally posted by len1
Well then, sodium salts you think dont, and it follows sodium carbonate is as good as the hydroxide. |
In my previous post I described exactly the opposite (again!). That is, when using Na2CO3 you get the decarboxylation of the type c
described in the previous post. Hence the main products are benzene and sodium phthalate in case of benzoic acid (or whatever else depending on the
exact conditions). When using NaOH you get the decarboxylation of the type d as long as there are at least 2 eq. NaOH. This gives
almost exclusively benzene (provided there is no O2 present). Clearly the reaction type c gives lower yields than reaction type
d. Under conditions for c the theoretical maximum conversion to benzene when using Na2CO3 is 50%, the other half
being lost as phthalate or terephthalate.
PS: Am I really so lousy in explaining things in English? Even though it's not my native language and I do not use it much, I find it hard to believe
I'm so incomprehensible. 
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
According to the attached paper, nicotinic acid can be decarboxylated by hydrothermolysis if one has an autoclave. Heating the aq. solution at 250°C
for 120h gives 52% conversion to pyridine. At the same conditions in the presence of one eq. of HCOOH the conversion increases to 97%.
Aqueous high-temperature chemistry of carbo- and heterocycles. 1. Introduction and reaction of 3-pyridylmethanol, pyridine-3-carboxaldehyde,
and pyridine-3-carboxylic acid
Alan R. Katritzky, Andrzej R. Lapucha, Ramiah Murugan, Franz J. Luxem, Michael Siskin, and Glen Brons
Energy & Fuels, 4 (1990) 493-498.
Attachment: Aqueous high-temperature chemistry of carbo- and heterocycles 1.pdf (781kB)
This file has been downloaded 1672 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
LSD25
Hazard to Others
  
Posts: 239
Registered: 29-11-2007
Member Is Offline
Mood: Psychotic (Who said that? I know you're there...)
|
|
Here is a route using copper chromite catalyst - seems rather simple:
| Quote: | Decarboxylation of Nicotinic Acid
Nicotinic acid (35 mg) was retluxed with quinoline (1 ml) and copper chromite (40 mg) for 20 min in a stream of nitrogen. The liberated carbon dioxide
was passed through a solution of 2 N sulfuric acid (to remove pyridine vapors), and then trapped in ethanolamine- methyl cellosolve or barium
hydroxide solution. The yield of carbon dioxide in the reaction was 90% of the theoretical yield. |
http://article.pubs.nrc-cnrc.gc.ca/ppv/RPViewDoc?issn=1480-3...
They report the yield of CO2 rather than pyridine which is wierd, although they wanted their radioactive carbon back
Of course, what seems rather less simple is preparing the copper chromite catalyst, would it be the one on orgsyn?
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2...
Here is another, more detailed look at the same reaction, which gives specific details:
| Quote: | Decarboxylation of nicotinic acid (I). An intimate mixture of nicotinic acid (20mg.) with copper chromite (100mg.) was suspended in silicone oil (MS
550) (1ml.) and placed in the apparatus shown in Fig. 3. The apparatus was flushed with dry C02-free N2, while the tube containing the decarboxylation
mixture was heated to 235C in a bath of silicone oil. The effluent gas was passed first through a 30ml. pear-shaped, double-necked flask cooled in
ice, as shown in Fig. 3, to collect the pyridine (XII), and then through a bubbler containing carbonate-free 2N-NaOH (3ml.) to collect the CO2.
Decarboxylation was complete after 1 hr. at 235°. Some of the pyridine, however, remained condensed in the delivery tube. This was washed into the
receiver by adding acetic acid (the solvent for the next conversion-see below) through the small entry port in the delivery tube.
|
http://www.biochemj.org/bj/102/0087/1020087.pdf (this came from page 6 of the PDF which also shows the apparatus).
PS The reason for the silica oil is explained on page 2 of the second PDF
| Quote: | Nicotinic acid (I) is decarboxylated efficiently by heating with coppear chromite, provided that certain precautions are taken. Sublimation and
decarboxylation of nicotinic acid both commence at 235C; even with a very slow gas flow rate, it is possible to drive nicotinic acid through several
inches of heated copper chromite supported on asbestos, with only slight decarboxylation. Consequently, the nicotinic acid was intimately mixed with
five times its weight of copper chromite catalyst and the mixture suspended in silicone oil.
Decarboxylation was complete after heating this mixture for lhr. |
[Edited, reaction temp was shown as 2350 and even silicon oil will struggle at that temp ] ]
[Edited on 16-6-2008 by LSD25]
Whhhoooppps, that sure didn't work
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have a plan for making pyridine that is relatively straightforward, except for one problem: aquisition of hypophosphorous acid. Being it is List I
makes this unobtainium for me. 
Anyway, here's the proposed synthesis:
1. Using the Hofmann hypobromite reaction, convert nicotinamide (OTC no-flush niacin) into 3-aminopyridine. There's even an orgsyn procedure for
this.
2. Diazatize the 3-aminopyridine. Then treat it with hypophosphorous acid. This removes the -NH2 group, leaving pyridine.
Those fortunate enough to live in a non-police state can likely still buy the acid. Then again you can probably buy pyridine easily also.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Ethanol and other alcohols also reduce ArN<sub>2</sub><sup>+</sup> to ArH + N<sub>2</sub>, though rarely in good
yields. In some cases however this method actually works almost as well as hypophosphorous acid, especially on some heterocyclic compounds which
3-aminopyridine is. According to a probable mechanism, isopropanol might work better than ethanol.
The Hoffman rearrangement of nicotinamide is supposedly described in WO2005070888 (NaOCl/NaOH 0°C->90°C; 86% yield).
[Edited on 7/7/2008 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
For those of you fortunate to live in place where methylated spirits is still denatured with pyridine and in possesion of a good still the following
procedure works nicely 
1) Add hydrochloric acid dropwise to the spirits until all the pyridine is converted into it's hydrochloride salt.
2) Distill off the pet ether and methanol.
3) Distill off an ethanol fraction for solvent use.
4) Add water and strip the remaining ethanol.
5) Crystallise out your pyridine hydrochloride.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
The following information was retrieved from:
http://customs.hmrc.gov.uk/channelsPortalWebApp/channelsPort...
Methylated spirits (UK composition)
90% vol. ethanol;
9.5% vol. 'wood naphtha'; and
0.5 vol. crude pyridine.
To each 1,000 litres of which is added:
3.75 litres of mineral naphtha (petroleum oil); and
1.5 grammes of methyl violet.
I am guessing that it should be 0.5% vol (as this completes the 100%) pyridine. That means that for 1ltr of distilled "meths" you can obtain (at
best!) 5ml of CRUDE pyridine. This is not an especially good way of obtaining pyridine, but I admit it is better than none.
However, upon further reading, 'wood naphtha' is defined as:
"There is no prescriptive list of ingredients, but some or all of the following are found in approved synthetic wood naphtha:
pyridine;
pyridine bases;
allyl alcohol;
crotonaldehyde;
picoline;
denatonium benzoate; and
methyl alcohol.
So there may well be more pyridine in there.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
I see the purification procedure for pyridine in the 3rd and 5th editions of Vogel and wonder just how dilute a solution of pyridine in ethanol with a
correspondingly smaller addition of ZnCl2 and HCl can be, to produce a precipitate in the freezer. I've been wondering this for quite a while but have
no way to test this, since there is no product denatured so sold here.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Have a look at ;
http://en.wikipedia.org/wiki/Methylated_Spirit
and
http://www.opsi.gov.uk/si/si2005/20051524.htm#5
Most modern UK methylated spirits use a substitute for wood naptha that consists of methanol and a marker.
I would guess that they pretty much use neat technical pyridine as well.
B & Q methylated spirits pretty much follows this formulation, go to;
http://www.diy.com/diy/jsp/bq/nav/nav.jsp?isSearch=true&...
and click on health and safety infomation
Raw methylated spirits has a distinct pyridine odour. On mixing methylated spirits with hydrochloric acid the result is a slighty opalescent liquid
with a strong smell of petroleum spirit.
|
|
|
jarynth
Hazard to Self
 
Posts: 76
Registered: 12-8-2008
Member Is Offline
Mood: No Mood
|
|
Q. How to tell whether the denatured alcohol contains pyridine? In this case, the concentration would be about 0.5-1%, according to composition info
found in this thread.
Here's a proposal. Possible nitrogen-containing co-denaturants include dyes and denatonium benzoate. A first strong distillation would get rid of
these high-boiling compounds, while passing most of the pyridine. The distillate is then acidified with aq HCl. A second disillation would separate
low-boiling compounds including ethanol, methanol, acetone, other alcohols and ketones, short alkanes, some water, etc, which constitutes a useful
solvent anyway. The contents of the flask are aqueous and can be washed with copious amounts of ether, which can then be retrieved almost
quantitatively by distillation for repeated use. The aqueous layer now only contains pyridine hydrochloride, which affords the pyridine by
basification and subsequent fractional distillation (or is there a quicker way?).
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
I completed some initial small scale testing for the production of Pyridine by decarboxylation of nicotinic acid with copper chromite.
The copper chromite was produced following the procedure outlined in Organic Syntheses, Coll. Vol. 2, p.142 (1943); Vol. 19, p.31 (1939). I opted to
use the crude material obtained after calcining of the barium copper ammonium chromate feed stock. The subsequent acid washings were omitted. Thus the
catalyst material obtained is reported to be: Cr2CuO4·CuO·BaCrO4
http://img390.imageshack.us/my.php?image=dsc05444ck2.jpg
A small scale retort was assembled from a test tube fitted with a stopper and a bent pipette functioning as an air condenser.
http://img145.imageshack.us/my.php?image=dsc05443vz1.jpg
Several runs were made to evaluate the effective proportion of crude chromites to C6H5NO2. It was reported in the paper by T.A. Scott that an excess
of cromite was used by weight. Since their goal was careful recovery of all carbon and not production of a reagent, it seemed obvious that a 5x excess
was not necessary for economy sake.
Procedure as follows. The nicotinic acid was ground in a mortar and pestle with the chromite until homogenous. This was then placed in the test tube
and stopper fitted with the condenser. The assembly was placed on a 45% angle in a clamp. The lower end was gently heated using a propane flame. The
sides of the tube were warmed when well into the reaction to effect the vaporization of the volatiles and carry them into the receiver vial. The first
two runs were done with silicon oil but the third was done to determine if it was even necessary and it was not. In this setup the sample size is
small enough to negate heat conduction issues.
The material collected was a mix of sublimated nicotinic acid and pyridine as identified by FTIR.
http://img529.imageshack.us/my.php?image=dsc05441lh9.jpg
The crude mix was weighed and then evaporated on a watch glass the re-weighed to determine the volatile proportion. Test results from 5 runs as
follows:
1) 2 gm nicotinic acid, 0.5gm mixed chromites, 4 ml. silicon oil -
solid residue: 0.66 gm. volatile residue: 0.186 gm.
2) 2 gm nicotinic acid, 1.0 gm mixed chromites, 4 ml. silicon oil -
solid residue: 0.117 gm. volatile residue: 0.806 gm.
3) 2 gm nicotinic acid, 1.0 gm mixed chromites -
solid residue: 0.056 gm. volatile residue: 0.804 gm.
4) 2 gm nicotinic acid, 1.5 gm mixed chromites-
solid residue: 0.069 gm. volatile residue: 0.863 gm.
5) 2 gm nicotinic acid, 1.75 gm mixed chromites-
solid residue: 0.044 gm. volatile residue: 0.868 gm.
Obviously working with such equipment in this manner introduced some errors but it is fairly certain that using pure copper chromite is not necessary
nor in such an excess for good yields.
Wiki says: "distinctively putrid, fish-like odour" I don't know if I would characterize it as fish like, but it sure smells bad.
[Edited on 12-9-2008 by ordenblitz]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice work ordenblitz. It's good to see you posting experimental work again.
I will be needing some pyridine, shortly I hope. I have been trying to decide whether to make it or buy it. Your successful results are encouraging
and I will likely give your synthesis a try.
Will it be difficult to purify the pyridine product? Can you form a salt out of the nicotinic acid and separate that out by
crystallization/filtration? Or is there a better way?
Edit: On rereading it seems that you are saying that the solid residue is nicotinic acid and that the volatile residue is pyridine. In this case it
seems that distillation is the obvious route to purifying the pyridine?
[Edited on 12-9-2008 by Magpie]
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Thanks. It has been a little too quiet in my lab in the past year but I have been messing about more recently. I am not in need of any pyridine
however I wanted some copper chromite for another project. Mid synthesis on that I remembered this thread and decided to try it purely for the fun.
The first thing that happens upon heating is the nicotinic acid starts to vaporize and then sublimate on the inner walls of the reaction chamber.
Shortly after pyridine also condenses on the inner surfaces and does, to a certain extent, dissolve and wash back the errant nicotinic acid. The
papers mentioned heat of about 230 deg starts the reaction. While things did begin to happen at about that temperature, it did take more heat then I
anticipated to drive to completion. As you would imagine the reacted carbonized mass is a very poor heat transfer medium so you have to pour on the
flame near the end. I’m not sure I would use your good glassware for this nor probably is an electric mantle up to the job. In my opinion using a
metal reactor would be preferable to glass. One could construct something from steel pipe that would serve well for this purpose. If it were
elongated and mounted vertically and on the lower end most heat be concentrated, with the upper part cooler the condensed pyridine should wash back
the sublimed nicotinic acid to the solid chromite effecting a better yield. A sealed reactor might be even better however there is some gas formed so
it may not be practical depending on how high the internal pressure would rise. As for cleaning the product, I think a simple distillation would be
all that is necessary.
Compared to my work with benzene from benzoates in my opinion this reaction is less trouble and has a better yield. With simple equipment and little
time invested one could generate quite a bit of pyridine. The only time consuming part was making the copper chromite although it’s not
complicated.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for the additional details and insights. I just finished ordering some (NH4)2Cr2O7 and will hopefully have my own homemade pyridine some day
soon.
Rant: I think it is important that we continue to develop home lab friendly procedures so as to be able to readily synthesize most of the basic (no
pun intended) reagents. With ever-increasing restrictions being placed on the market place by our governments we home chemists must become evermore
self-reliant. Some may ask why make pyridine when it is so commonly available. Well, when you add in the hazmat charge it becomes fairly expensive
for one thing. And even if the reagent is not restricted you often times have a nagging feeling that by ordering it your name will be placed on some
government list. Besides, making your own is always so much more satisfying, not only intellectually, but for the revenge it provides against stupid
legislators, bureaucrats, and LE listmakers. End rant.
That's why your success is especially pleasing to me. 
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
I decided since I have a lot of the required intermediary copper chromite material I would try this reaction again scaled up and in glass. Damage was
possible to the boiling flask but I have a few, so was prepared to sacrifice one for the cause. I was in fact completely wrong about the temperatures
required and the possible heat transfer issues of the starting mix.
In a 50 ml flask was placed 24 grams of nicotinic acid intimately mixed with 12 grams of Cr2CuO4·CuO·BaCrO4 catalyst mix.
http://img75.imageshack.us/my.php?image=dsc05446ni4.jpg
http://img222.imageshack.us/my.php?image=dsc05449ue1.jpg
One should definitely start by warming this mix slowly because it will limit or avoid all together much of the sublimation of the nicotinic acid.
http://img222.imageshack.us/my.php?image=dsc05451od1.jpg
A little heat blanket is handy here to speed things along.
http://img217.imageshack.us/my.php?image=dsc05466yf6.jpg
I got in a hurry though and turned the mantle all the way up in the beginning thinking I would not have enough heat. I had to unplug and let the
moving air of the hood cool things a little. You can see the white smoke of nicotinic acid filling the flask and condenser.
http://img68.imageshack.us/my.php?image=dsc05452ky8.jpg
After things got settled down and a little cooler, it really only took a setting of about 50% on my controller to keep the reaction running smoothly.
This lower temperature allowed some pyridine to reflux into the mix washing back any escaped C6H5NO2. You can see small droplets forming here but
really no white fumes indicating sublimed material. I did not measure the temperature of the reactants but I guess the flask to be at less then 250
deg at this point and working well.
http://img134.imageshack.us/my.php?image=dsc05480xn6.jpg
At this point I was collecting about a drop every 6 to 8 seconds. Total time of the reaction was about 40 minutes pushing it slowly. With set up and
takedown all told about an hour and a half of lab time.
http://img225.imageshack.us/my.php?image=dsc05461gm8.jpg
The result was 11.8 grams of a fairly clean first run product. I will distill the pyridine after I have made a few more runs to perfect the technique
at this size.
http://img225.imageshack.us/my.php?image=dsc05487rm4.jpg
I have to say that this reaction in standard glassware is very simple and easy to do. My presumption that the mantle could not generate nor the glass
stand the temperatures required was completely incorrect. Also my assertion that heat transfer through the reactants would be difficult but this was
also not the case. Yields are good and the reaction runs smoothly and controllably. If you take your time during the initial heat up sublimation of
the nicotinic acid will be very minimal.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Sweet! You have basically given us a home lab friendly procedure for making pyridine. Thank you!
I did try to make pyridine by decarboxylation of nicotinic acid with NaOH using dry distillation. I made mostly carbon and just enough pyridine to
stink up the lab.
I'm curious as to the mechanism of the copper chromite catalysis. I wonder if it is known?
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
LSD25, I believe, found this procedure. I just did the easy part.. mixed stuff up!
Have to say though.. it was pretty simple to do all told.
Now... what to use the pyridine for?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
| Quote: |
Now... what to use the pyridine for?
|
I plan to use a little in the synthesis of cinnamic acid, where it has been shown to be a catalyst.
I would think it could be used to make pyridium chlorochromate (PCC), useful for making aldehydes from alcohols. Seems like I've seen a procedure for
making PCC somewhere.
|
|
|
jarynth
Hazard to Self
 
Posts: 76
Registered: 12-8-2008
Member Is Offline
Mood: No Mood
|
|
Very helpful data ordenblitz! And congratulaton on the experimental feel of trying several runs. Can you tell us about the recovery of the catalyst?
Did you try it at all? Making the catalyst requires quantitative amounts of dichromate, which - if wasted - would drive up horribly the cost of making
pyridine by this route (not to speak of the necessary expense: the cost of nicotinic acid). Clearly the cations can be recovered by brute force, but
how about cleaning the tarry residues off the chromites without degrading them?
Copper salts are famous for catalyzing decarboxylation in general. I tried heating a small mixture of nicotinic acid with 1) slightly oxidized copper
powder, and 2) copper sulfate, in a test tube, on an open flame. 1) only gave a white sublimate and no fishy smell before carbonizing, whereas 2)
produced small droplets and almost no sublimate, and a light fishy/tarry smell was noticeable. Unfortunately this dripped back onto the solids and was
impossible to extract once cool.
[Edited on 9-10-2008 by jarynth]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Today I made some pyridine following the full scale method of ordenblitz. I must say that it worked very well. At first I tried the small scale
method but problems with sublimation of the nicotinic acid, etc, resulted in failure.
For the full scale method I followed the procedure of ordenblitz to the letter, right down to the paper clip holding the insulating blanket. I
brought up the heat very slowly, and never set the voltage controller over 65% for my 80 watt Glas-Col heater. The pyridine would come over in spurts
of about 10 drops every minute at the peak of production. This slowed as the run progressed. I didn't measure the volume of product but it looked
the same as that of ordenblitz. This would place the yield at very near the theoretical 13.1 ml. I didn't see any sublimation above the powder so
guess that any contamination of the product would likely be a small amount of water. A picture of the distillation set up is shown below. A 2nd
picture is shown in the following post.
It's Halloween - pyridine for everyone! 
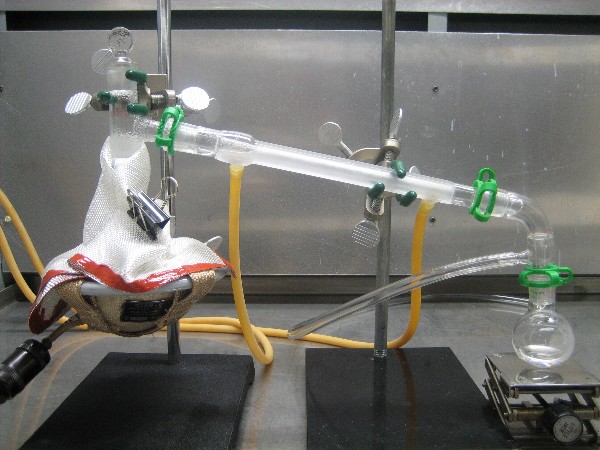
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's a picture of the powder following the run. You can see the sublimated nicotinic acid as a hoarfrost on the surface. This powder broke up very
easily and there was no damage to the RBF.

|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
If you want to make a significant quantity of it, niacin will be too expensive.
The industrial synthesis is much more applicable and, for a change, doesn't require any insane pressures, temperatures or catalysts.
However, the industrial synthesis does not produce pure pyridine as I understand it. If you do some searching, you'll find it produces a number of
lines based on pyridine, as well as the pure product it's self. In some work, I saw around 50% suggested as the pure pyridine yield - that was with a
catalyst.
I've yet to find one that's optimized to produce pure pyridine. If anyone does know of one, give me a shout (u2u).
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Nice work magpie! I guess I missed your post two weeks ago. As always nice setup and nice clean lab.
@ peach you might want to check out this website; some good prices on supplements in large quantity
http://purebulk.com/niacin-usp-c-65.html?zenid=da86794355fca...
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
| Pages:
1
2
3
4 |