| Pages:
1
2 |
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Pentryl
<center><font size="10">Pentryl</font>
<font size="5">(Trinitrophenylnitraminoethyl Nitrate)</font>
<b>By Axt</b></center>
This study was conducted to determine the easiest way to produce the explosive, pentryl, using backyard methods. It was also done to synthesize useful
intermediates that could be used for other purposes. It was found that pentryl could be produced in five quite easy steps starting from sodium
benzoate, which was decarboxylated to benzene. The benzene was then chlorinated to chlorobenzene and the chlorobenzene nitrated to
2,4-dinitrochlorobenzene. The fourth step was to condense 2,4-dinitrochlorobenzene with ethanolamine to produce 2,4-Dinitrophenylaminoethanol which
was then nitrated to pentryl.
All the precursors were purposefully sourced from readily available products available in supermarkets, hardware stores and farm supply merchants. The
properties of the precursors and intermediates for the synthesis of pentryl are listed in tables 1 and 2.
<center><b>Table 1: Physical Properties of Pentryl Precursor</b>
<img src="http://www.sciencemadness.org/scipics/axt/precursor-properties-table.jpg"></center>
<center><b>Table 2: Physical Properties of Pentryl Intermediates</b>
<img src="http://www.sciencemadness.org/scipics/axt/intermediate-properties-table.jpg"></center>
<font size="5">Benzene</font>
Benzene synthesis was studied in detail by other sciencemadness.org members[13]. Benzene is conveniently made by the decarboxylation of sodium
benzoate with sodium hydroxide[1] (figure 1). The dry distillation was done using an apparatus made from copper plumbing pipe and fittings with a
metal can (similar to a paint can) used for the heating vessel (figure 2). Within the can was placed a number of lengths of copper pipe to act as a
conduction aid.
<center><img src="http://www.sciencemadness.org/scipics/axt/benzene-scheme.jpg">
<i>Figure 1: decarboxylation of sodium benzoate to benzene</i></center>
<i>Precursors</i>: The sodium benzoate was sourced from a food supply store and came in 1kg bags. Sodium benzoate is used as a
preservative for the prevention of yeast, bacteria and fungi. As an ingredient it’s mainly used in baking powder, fruit esters, antiseptic,
winemaking and effervescent beverages. Sodium hydroxide was purchased from the local hardware store in a 3kg container under the name “caustic
soda”. Its main domestic use is as a drain cleaner where it’s able to saponificate the grease and decomposes the protein of hair to unblock
drains.
<i>Synthesis of Benzene</i>: A powdered mixture comprised of 150g sodium benzoate and 75g sodium hydroxide was poured over and into the
copper pipes in the metal can and attached to the distillation apparatus. After about 10 minutes of strong heating on a gas burner, an orange
distillate started to collect in the receiving flask. This was continued for about 45 minutes by which time 83ml of crude benzene had been collected
which is 90% of the theoretical yield. Redistilling the crude orange benzene by heating in a boiling water bath gave a clear distillate leaving behind
a small quantity of an orange high boiling point product composed predominantly of biphenyl[1]. The final yield of clear benzene was 75ml or 81% of
theory.
<center><img src="http://www.sciencemadness.org/scipics/axt/benzene-distillation.jpg">
<i>Figure 2: Benzene distillation apparatus</i></center>
<font size="5">Chlorobenzene</font>
The chlorination of benzene using trichloroisocyanuric acid (TCCA) was first described by Juenge, Beal and Duncan[2], it was found that in the
presence of anhydrous ferric chloride or 50% sulphuric acid catalysts, benzene could be converted to chlorobenzene in high yield (based on TCCA used)
with precipitation of cyanuric acid as a byproduct (figure 4). This is analogous to the reaction of benzene with molecular chlorine, which requires
Lewis acid catalysis. I simplified and modified their procedure to provide a higher yield based on the benzene used.
<center><img src="http://www.sciencemadness.org/scipics/axt/chlorobenzene-scheme.jpg">
<i>Figure 4: Chlorination of benzene with TCCA</i></center>
<i>Precursors</i>: Trichloroisocyanuric acid (TCCA) also known as “symclosene” or
“1,3,5-Trichloro-s-triazine-2,4,6(1H,3H,5H)-trione” was bought as swimming pool chlorinator tablets in a hardware store, it is used to reduce
algae and other microorganisms in swimming pool water. The product used was sold under the name “HY-CLOR” and marked “850g/kg available chlorine
present as trichloroisocyanuric acid”. Sulphuric acid is available at hardware stores as drain cleaner, I used a product called “Mo-Flo” and was
marked “1835g/kg sulphuric acid”.
<i>Synthesis of Chlorobenzene</i>: 75ml of benzene was added to 50ml of water and 50ml sulphuric acid in a 300ml conical flask. 75g of
finely powdered TCCA was then swirled into the solution. The mixture was then heated to 60-80°C for 8 hours (figure 5).
<center><img src="http://www.sciencemadness.org/scipics/axt/chlorination-ap.jpg">
<i>Figure 5: Benzene chlorination</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/purification-cb.jpg">
<i>Figure 6: Crude distillate (left); after washing (centre); after boiling (right)</i></center>
The slurry was then cooled and transferred into a metal can for distillation, where it was heated at 130°C until the distillate stopped coming over.
This gave 54ml of a dense milky yellow organic layer (d 1.14g/cm2) underneath a green aqueous solution of copper chloride from the reaction of HCl and
Cl2 with the copper condenser (figure 6). The organic layer was separated and washed with dilute sodium hydroxide to remove dissolved Cl2 and HCl,
then with water to give 48ml of a milky white liquid (figure 6). The milky liquid was then quickly heated to 110°C where it boiled vigorously;
temperature of the liquid was allowed to rise to 130°C then quickly cooled to yield 34ml of clear chlorobenzene (figure 6), for a final yield of 40%
of theory based on benzene used.
<font size="5">2,4-Dinitrochlorobenzene</font>
The nitration of chlorobenzene yields 2,4-dinitrochlorobenzene (DNCB) (figure 7), a reactive intermediate to many explosives some of which have been
adopted for military and civilian applications. Examples of other explosives that can be derived from DNCB are:
<u>Tetryl</u>: By condensing with methylamine followed by nitration in mixed acids.
<u>Picramide</u>: By condensing with ammonia, then nitration.
<u>2,4-Dinitrobenzenediazonium Perchlorate</u>: By condensing with ammonia followed by diazatisation then precipitation with perchloric
acid.
<u>Picric acid</u>: By reacting with sodium hydroxide in water, then nitration.
<u>Trinitroanisole</u>: By reacting with sodium hydroxide in methanol, then nitration.
<u>Hexanitroazobenzene</u>: By condensing with hydrazine, then nitration.
<u>Hexanitrodiphenylamine</u>: by condensing with aniline, then nitration.
<center><img src="http://www.sciencemadness.org/scipics/axt/dncb-scheme.jpg">
<i>Figure 7: Nitration of Chlorobenzene to DNCB</i></center>
<i>Precursors</i>: Potassium nitrate was obtained from an agriculture supply store as fertilizer, the product came in a 25kg bag marked
“K-Nitrate” and coded 13-0-38.
<i>Synthesis</i>: The synthesis is described by Davis[3] which I adapted to use mixed KNO3/H2SO4 acids instead of the concentrated
HNO3/H2SO4. The nitration mixture was made by combining 80.9g (0.8mol) potassium nitrate with 130ml 98% sulphuric acid, this was dissolved then cooled
to 25°C. With rapid stirring 20.4g (0.2mol) chlorobenzene was then added in small portions, making sure to keep the resultant exotherm from heating
the mixture over 50°C. After the DNCB is added the temperature was slowly raised to 95°C and stirred at this temperature for 2 hours.
Once taken off the heat the DNCB separated as a light yellow upper layer (figure 8), after cooling this solidifies. The nitration mixture was then
stirred into 1 litre of cold water which separated the DNCB as chunks that fell to the bottom (figure 8). The acidic aqueous solution was then
decanted and replaced with 750ml water, which was boiled to melt the DNCB; this was rapidly stirred to wash the DNCB of any remaining acids. The water
was then decanted and the DNCB powdered and dried. Yield was 32g or 79% of theory.
<center><img src="http://www.sciencemadness.org/scipics/axt/dncb-precip.jpg">
<i>Figure 8: Nitration of chlorobenzene (left); washing of dinitrochlorobenzene (right)</i></center>
<font size="5">2,4-Dinitrophenylaminoethanol</font>
DNCB condenses readily with ethanolamine to yield 2,4-Dinitrophenylaminoethanol (figure 9). This condensation step has been studied and published by a
number of workers such as Clarke[4], and Kremer[9] whom studied the condensation of mononitrochlorobenzenes with ethanolamine and also some analogous
reactions[10].
<center><img src="http://www.sciencemadness.org/scipics/axt/dnpae-scheme.jpg">
<i>Figure 9: Synthesis of 2,4-Dinitrophenylaminoethanol</i></center>
<i>Precursors</i>: Ethanolamine was available in a product called “Selleys BBQ Kleen” and is an ingredient in many grease cleaners;
BBQ Kleen was 10.5% ethanolamine though also contained Polyoxyethylene lauryl ether as a detergent, dispersant and surfactant[8]. The impurities were
removed by boiling the solution until the temperature rose to the boiling point of ethanolamine. The ethanol used was “methylated spirits” bought
from hardware store as a 95% solution.
<i>Synthesis</i>: Into 145g Ethanol was added 8.6g ethanolamine and 28.4g DNCB. The solution was then heated with stirring to 70°C until
the DNCB dissolves, the solution turned orange. With rapid stirring 5.7g sodium hydroxide in 8.5g water was added slowly whereby the solution turned
dark cherry red and of sodium chloride precipitate formed. The solution was left at 70°C for 30 minutes then filtered, the precipitate is kept as it
contains bright yellow bis(dinitrophenyl)aminoethanol.
The filtrate was boiled down to 50ml and let stand, orange crystals formed, the supernatant liquid was decanted and the orange crystals of
2,4-Dinitrophenylaminoethanol were flushed with cold ethanol then dried (figure 10). Yield was 15g (47% of theory)
<center><img src="http://www.sciencemadness.org/scipics/axt/dinitrophenylaminoethanol-crystals.jpg">
<i>Figure 10: crystals of 2,4-dinitrophenylaminoethanol</i></center>
The original precipitate that formed was then dissolved into 400ml water, this dissolved the sodium chloride leaving the bright lemon yellow byproduct
of bis(dinitrophenyl)aminoethanol (figure 11), which was filtered and dried.
<center><img src="http://www.sciencemadness.org/scipics/axt/bdnpae-precip.jpg">
<i>Figure 11: precipitate of bis(dinitrophenyl)aminoethanol</i></center>
<font size="5">2,4,6-Trinitrophenylnitraminoethyl Nitrate (Pentryl)</font>
Pentryl is a powerful explosive, first produced by Moran[6] by the nitration 2,4-dinitrophenyleneaminoethanol (figure 12). Another route was also
patented by von Herz[7] by the nitration of phenylaminoethanol obtained by reacting aniline with ethylene oxide or ethylene glycol chlorohydrin.
Pentryl is of novel interest due to it containing the three major energetic moieties, the nitro, nitrate and nitramine groups on a single compound.
<center><img src="http://www.sciencemadness.org/scipics/axt/pentryl2-scheme.jpg">
<i>Figure 12: Nitration of 2,4-dinitroaminoethanol</i></center></i></center>
<i>Synthesis</i>: A nitration mixture was made by dissolving 54.6g (0.54mol) potassium nitrate into 100ml sulphuric acid then cooled to
25°C. 13.6g (0.06mol) 2,4-Dinitrophenylaminoethanol was then dissolved with rapid stirring into the nitration mixture which raised in temperature to
50°C then suddenly solidified as the pentryl precipitated from the solution. The pasty mixture was then stirred into 1 litre of cold water, filtered
and washed with more water. It was then washed with dilute sodium bicarbonate solution, then a final wash with more water. The product was a fine
cream coloured powder. Recrystalisation from acetone/ethanol yielded clear-yellow crystals (figure 13). Final yield of pentryl was 18.2g or 84% of
theory.
<center><img src="http://www.sciencemadness.org/scipics/axt/pentryl-tricrystals.jpg">
<i>Figure 13: Crude precipitate of pentryl (left, centre); after recrystalisation (right)</i></center>
<i>Explosive Properties</i>: The explosive properties of pentryl rate it highly amongst in the family of aromatic explosives containing
the trinitrobenzene moiety; comparative properties are listed in table 3. The pentryl made in this study was pressed into a drinking straw and
detonated against an aluminium plate (figure 14). Lead block compression tests were conducted by Clarke[4] (figure 15).
<center><b>Table 3: Properties of Aromatic Explosives</b>
<img src="http://www.sciencemadness.org/scipics/axt/properties-table.jpg"></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/pentryl-plate.jpg">
<i>Figure 14: detonation of pentryl against aluminium plate</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/lead-block-compression.jpg">
<i>Figure 15: Lead block compression tests</i></center>
<font size="5">Bis(2,4,6-trinitrophenyl)aminoethyl Nitrate (HNDPAN)</font>
The byproduct of the DNCB/ethanolamine condensation, bis(dinitrophenyl)aminoethanol, can be nitrated to the explosive
bis(2,4,6-trinitrophenyl)aminoethyl Nitrate (figure 15).
<i>Synthesis</i>: Two solutions were made, one by dissolving 12g (0.12mol) potassium nitrate in 42g sulphuric acid and another by
dissolving 2g (0.005mol) bis(dinitrophenyl)aminoethanol into 20g sulphuric acid. With both solutions cooled to 25°C they were slowly combined by
pouring the bis(dinitrophenyl)aminoethanol solution into the potassium nitrate solution. A slight exotherm resulted and the solution thickened and
turned from yellow to cream as the HNDPAN precipitated. The HNDPAN was stirred into 700ml cold water then neutralized with dilute sodium bicarbonate
solution. After a final wash with water it was filtered and dried to yield 2.31g (86%) of HNDPAN.
<center><img src="http://www.sciencemadness.org/scipics/axt/hndpan-scheme.jpg">
<i>Figure 16: Nitration of bis(dinitrophenyl)amino ethanol to HNDPAN</i></center>
<i>Explosive Properties</i>: The explosive properties of HNDPAN are listed in table 1. That made in this study was not detonated, however
when ignited it deflagrated very vigorously with a large orange flame and grayish smoke (figure 17).
<center><img src="http://www.sciencemadness.org/scipics/axt/btnpaen-1.jpg">
<i>Figure 17: deflagration of HNDPAN</i></center>
<b>References</b>
1] J. F. Norris “Experimental Organic Chemistry”; 2nd edition; pg. 132; McGraw-Hill Book Company, New York & London. (1924)
2] E. C. Juenge, D. A. Beal & W. P. Duncan. “Chlorination of aromatic systems with trichloroisocyanuric acid under polar and free-radical
conditions”; Journal of Organic Chemistry; 35(3); 719-722. (1970)
3] T. L. Davis “The Chemistry of Powder and Explosives” Pg. 141; New York, John Wiley & Sons; London, Chapman & Hall. (1941)
4] Le Roy V. Clarke “Analogues of Tetryl I. Trinitrophenylnitraminoethyl Nitrate (Pentryl)” Industrial and Engineering Chemistry; 25(12); pg.
1385-1390; (1933)
5] B.T. Fedoroff et. al. “Encyclopedia of Explosives and Related Items“. vol. 1; pg. A425. Picatinny Arsenal; New Jersey; USA (1960)
6] R. C. Moran “Explosive and Process of Making Same” US patent #1560427 (1925)
7] E. von Herz “An Improved Method of Producing Explosives” British patent #367713 (1931)
8] “MSDS - Selleys BBQ Kleen” SH&E Shared Services, Orica; Australia.
9] C. B. Kremer “Alkanolamines. II. Reaction of the Chloronitrobenzenes with Monoethanolamine” Journal of the American Chemical Society; 59(9);
1681-1682; (1937)
10] C. B. Kremer & M. Meltsner “Intermediates of Pentryl Analogs. Chloronitroanilino Alkanols” Journal of the American Chemical Society;
64(6); 1285-1286; (1942)
11] Le Roy V. Clarke “Analogues of Tetryl. Hexanitrodiphenylaminoethyl Nitrate” Industrial and Engineering Chemistry; 26(5); pg. 554-557; (1934)
12] Dobratz, B and Crawford, P. "LLNL Explosives Handbook - Properties of Chemical Explosives and
Explosive Simulants" Lawrence Livermore National Laboratory. California. (1985)
13] Chromium, with comtributions by IPN, Ordemblitz, S.C. Wack, and Organikum "Preparation of Benzene" (online) http://www.sciencemadness.org/member_publications/benzene_pr...
[Edited on 24-12-2007 by Axt]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Nice work!
I like the witness plate, it really shows the different brisances between the petryl and the cap.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
PDF version is now available for previewing:
http://www.sciencemadness.org/member_publications/pentryl.pd...
I had two comments: first, I think there should be a warning about 2,4 DNCB's action as a contact sensitizer; the burden ultimately rests with the
experimentalist to understand his compounds' risks, but I think the document itself should at least mention this. You don't need to write anything; I
will gladly add a note myself if you do not object.
I noticed that the sulfuric acid was reported as 1835 g/kg. This doesn't make much sense; surely it's supposed to be 1835 g/L?
As usual for you, this was an impressive and thorough document of amateur experimentation.
PGP Key and corresponding e-mail address
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Add my kudos to the others .
Nice work Axt .
An OTC route to pentryl is pretty ballsy .
I can't help but wonder what might happen
if paradichlorobenzene moth crystals were dinitrated ,
and reacted with ethanolamine .....would it react
the same on both the chlorines , and add the second
side chain there to form an intermediate for a
a possible " Heptryl " ????
Also I wonder if chlorinated toluene or xylene might substitute for the benzene ....to perhaps yield ring
substituted methyl variants on pentryl ...or some
mixed isomers which may be low temperature castable or even liquid mixtures at ordinary temperatures .
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
if paradichlorobenzene moth crystals were dinitrated |
Sounds like a major pain in the ass requiring pretty extreme conditions.
Nice work Axt.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Axt, that is one excelent work you did. Congratulations!
| Quote: | Originally posted by Rosco Bodine
I can't help but wonder what might happen if paradichlorobenzene moth crystals were dinitrated , and reacted with ethanolamine .....would it react the
same on both the chlorines , and add the second side chain there to form an intermediate for a possible " Heptryl " ???? |
Dinitration of p-dichlorobenzene yields a mixture of two regioisomers, 2,5-dinitro-1,4-dichlorobenzene and 2,6-dinitro-1,4-dichlorobenzene of which
apparently the first prevails (I uploaded a paper describing this nitration somewhere in the Energetic materials forum section).
2,5-dinitro-1,4-dichlorobenzene is able of reacting with ethanolamine or other such nucleophiles but is considerably less reactive than
2,4-dinitrochlorobenzene. Harsher conditions would be required and the resulting product would be N-(4-chloro-2,5-dinitrophenyl)aminoethanol.
No doubly substituted product should form since the amino group has a +M electrondonating effect and thus deactivates the aromatic ring for further
nucleophilic aromatic substitution.
On the other hand, 2,6-dinitro-1,4-dichlorobenzene should be even more reactive than 2,4-dinitrochlorobenzene as it has the p-chloro group in addition
of the two ortho-nitro groups. But again the product would be monosubstituted, that is N-(4-chloro-2,6-dinitrophenyl)aminoethanol. The other
chlorine group can not be substituted at all since that position is not activated for the nucleophilic aromatic substitution.
Therefore, for what regards the potential use of p-dichlorobenzene in similar explosives syntheses, only
N-(4-chloro-2,5-dinitrophenyl)aminoethanol which can be can be O- and N-nitrated as well as nitrated on the ring (on position 6) might be of
interest. The resulting coumpound should be N-(4-chloro-2,3,6-trinitrophenyl)-N-nitro-aminoethyl nitrate ( SMILE code:
O=[N+]([O-])c1c(c(c(cc1Cl)[N+]([O-])=O)N(CCO[N+]([O-])=O)[N+]([O-])=O)[N+]([O-])=O ). However the inert Cl is only dead weight and its only positive
property might only be in the density increase.
| Quote: | | Also I wonder if chlorinated toluene or xylene might substitute for the benzene ....to perhaps yield ring substituted methyl variants on pentryl ...or
some mixed isomers which may be low temperature castable or even liquid mixtures at ordinary temperatures . |
Toluene gets chlorinated to 2-chloro and 4-chloro toluene where the 2-chlorotoluene generally prevails somewhat.
The 2-chlorotoluene would probably give 2-chloro-4,6-dinitrotoluene from dinitration. This would be inert toward ethanolamine as the chlorine is not
activated for nucleophilic aromatic substitution.
The 4-chlorotoluene would probably yield 4-chloro-2,6-dinitrotoluene which has the similar problem of the chlorine on an non-activated position.
Ortho- and para-xylenes would, upon chlorination and dinitration, yield products that could arylate ethanolamine, but except for a few minor
regioisomeric nitration sideproducts there would be no position left for the trinitration of the ring.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
So no joy on those ideas  hmmmm...... hmmmm......
what about a chlorination of naphthalene ,
followed by nitration and subsequent reaction
with ethanolamine ?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
The condensation of 2,4-dinitrochlorobenzene with ethanolamine is most interesting.
There is the potential to use hydrazine instead of ethanolamine, which would give 2,4-dinitrophenylhydrazine, a very important reagent in organic
chemistry for testing the presence of a ketone or aldehyde. I might publish this synthesis in the future.
2,4-DNPH is useful for removing the denaturant from commercial ethanol denatured with MEK for production of high-purity ethanol. The resulting
MEK-dinitrophenylhydrazone can be cleaved and the DNPH recycled.
The chlorination of benzene using TCCA is interesting, but offers no advantage over the chlorination with elemental chlorine except the obvious
simpler apparatus and less danger associated with the reagents.
The lewis acid catalyst required for chlorination of benzene can be as simple as a little iron powder in the (dried) benzene which forms anhydrous
FeCl3 in situ.
[Edited on 26-2-2007 by garage chemist]
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
@ Davstar: In defence of pentryl, it was pressed to a much lower density then the PETN in the detonator and thats the effect you are seeing. Bit of
trivia, pentryl has the same oxygen balance & density as RDX. I couldnt find any VOD figures for pentryl at its higher densities, only @ 1.6g/cm3.
@ Polverone: yep you are of course correct about the g/kg->g/L, and yep you can add a warning about DNCB. Personally i didnt get the itching its
reported to produce however i couldnt rub my eyes for a week after without the sensitive skin around them burning, no matter how many times i washed
my hands, clothes whatever! I made a couple mistakes in tables and forgot to reference explosive properties, give me couple days and i'll fix that,
sorry.
@ Roscoe: There is Nonyl (trinitrobenzene with three nitraminoethyl nitrate moieties stuck to it!) which out performs pentryl. Though OTC it would be
a marathon 8 steps. I attaching references to other analogues. Naphthalene is monochlorinated with TCCA in 1 position, which you could probably
distill directly off TCCA as its a low BP liquid. I guess it would form an analogue if first nitrated in the 2,4 positions, though the conditions for
tetranitration of naphthalene may be a bit harsh for the nitraminoethyl nitrate moiety.
@ Garage Chemist: Not using a gas is a big advantage, also prevents the gas from evaporating off the volatile benzene. How selective is
benzene/Cl2/FeCl3 chlorination? TCCA will only ever produce monochlorobenzeneas it seems to form a complex between benzene and TCCA as an intermediate
with wont form with chlorobenzene. Oh, and you'd prolly use TCCA to produce the Cl2 anyways.
[Edited on 27-2-2007 by Axt]
Attachment: pentryl references 2.zip (1.4MB)
This file has been downloaded 1862 times
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Journal references used.
Edit: uploading or file size problems.. grrrr
Heres some of them
[Edited on 27-2-2007 by Axt]
Attachment: pentryl references.zip (953kB)
This file has been downloaded 2392 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I admit it....I have a thing for high brisance. Something about it just does it for me.

Any sand crush data around?
The monochlorination of benzenes with TCCA is also very interesting, expecially because I like to avoid the use of chlorine indoors with my current
ventilation.
Would the condensation work on other conjugated ring systems? Like...oh say a halotetrazole?
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
The document has been updated with the proposed minor changes and added to the member publications index.
PGP Key and corresponding e-mail address
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | | <u>Picric acid</u>: By reacting with sodium hydroxide in methanol, then nitration. |
Thats an error, it was meant to be:
<u>Picric acid</u>: By reacting with sodium hydroxide in water, precipitating with HCl followed by nitration.
<u>Trinitroanisole</u>: By reacting with sodium hydroxide in methanol, then nitration.
I was playing around with it because I couldn't actually find reference to picric acid from 2,4-dinitrochlorobenzene. But presumably it can be done by
heating DNCB in aqueous NaOH then precipitating from sodium salt with HCl, then nitration to picric. Can anyone confirm this?
|
|
|
Joeychemist
Hazard to Others
  
Posts: 275
Registered: 16-9-2004
Location: Canada
Member Is Offline
Mood: Sedated
|
|
heres one...
Attachment: US1299171 Manufacture of Picric Acid From Dinitrochlorobenzene.pdf (178kB)
This file has been downloaded 1931 times
|
|
|
Joeychemist
Hazard to Others
  
Posts: 275
Registered: 16-9-2004
Location: Canada
Member Is Offline
Mood: Sedated
|
|
...and the second
Attachment: US3283011 Preparation of Nitrophenols.pdf (314kB)
This file has been downloaded 2660 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
trinitrophenoxyethyl nitrate
Here's another derivative which is similar .
The material is described as intermediate between
picric acid and tetryl in sensitivity and power ,
~122% of TNT .
The precursor for nitration is derived from reaction of
dinitrochlorobenzene with ethylene glycol . It seems to me that other nitrated chlorinated aromatics might also
give analogous intermediates for further nitration .
The patent mentions dinitrochlorotoluene specifically
as workable in a similar reaction , and also states that
other polyhydric alcohols may condense with the nitrated
chloroaromatic compound to form intermediates which
may be further nitrated .
Perhaps this is a general scheme workable for condensing glycerin , erythritol , or pentaerythritol , sorbitol , ect. with an
assortment of various nitrated chlorinated aromatics , to form intermediates whose higher nitration end products could have desirable properties .
PATR Vol.8 , page P236 , states that the most basic precursor , glycol monophenyl ether , can also be obtained from reaction of dichlorobenzene with
ethylene glycol . If paradichlorobenzene works for this , then moth crystals and antifreeze may give the intermediate for nitration , in
a simpler way than having to first produce benzene and then chlorinate it and then dinitrate it .....which would eliminate much work .
If this is accurate information reported by PATR , the same
generality may also apply with respect to other polyhydric
alcohols as well , providing a whole new potential use
for moth crystals and various sugar alcohols  for for
producing new candidates for nitration .
I haven't even drawn out any of these reactions yet .....
I just now discovered this information and recognized its
potential usefulness .
I am still sorting out the nomenclature for this glycol monophenyl ether , trying to identify if B-phenoxyethanol ,
phenylethyleneglycol ether , and ......
2-phenoxyethanol , ( phenyl cellosolve ) are identical
compounds , so I can try to find the synthesis from
dichlorobenzene and ethylene glycol .
[Edited on 23-4-2007 by Rosco Bodine]
Attachment: US1560426 Trinitrophenoxyethyl Nitrate.pdf (161kB)
This file has been downloaded 2152 times
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Sounds like a mixup to me dichlorobenzene <-> dinitrochlorobenzene.. though it'd be nice if it were not. I bought p-DCB as bin deodoriser but am
yet to find a use for it.
The condensation product with ethanol is interesting as well, so DNCB/NaOH/EtOH gives 2,4-dinitrophenetole, and 2,4,6-trinitrophenetole on nitration.
Was used it as an effective TNT substitute, MP: 76-78°C and about as powerful. Also forms eutectic with trinitroanisole with melting point as low as
40°C. So by using OTC "methylated spirits" a lower MP would likely be obtained.
Good to get an extensive list with references to all these DNCB derivatives.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Actually I think that because there is more than one brief reference in PATR regarding the non-nitrated phenoxyethanol as a precursor capable of being
nitrated to both a dinitro-nitrate and also the trinitro-nitrate ,
that at least this much of the PATR article is correct .
It follows then that your pentryl described above might possibly be also produced from a non-nitrated precursor as well . Or at least a structurally
similar 5 nitrated compound , which could be a trinitro-dinitrate , three aromatic nitros , plus two of more nitroester nitros from nitration of
hydroxyls of a polyhydric alcohol having been condensed with an aromatic ring by whatever means .
The brief part of the PATR reference concerning the dichlorobenzene reactant is something which I also thought could be a typo , ( but not yet having
checked this out , I am keeping my fingers crossed that it is accurate , since that would be a wonderful development if it does prove to be true  ) These " hopeful " teasing sort of errors are scattered about in PATR . I hope
this isn't yet another PATR misprint . But I have to wonder what would magically remove the second ring chlorine from a condensate involving para . ) These " hopeful " teasing sort of errors are scattered about in PATR . I hope
this isn't yet another PATR misprint . But I have to wonder what would magically remove the second ring chlorine from a condensate involving para .
I also have my smaller reservations about whether a fully non-nitrated precursor compound can withstand nitration
of the aromatic ring and plural side chain hydroxyls simultaneously , in a one pot - one step nitration , which would be a highly exothermic nitration
and subject to oxidation . But I think it could be possible with good cooling .
The theoretical aspects of the possible para DCB condensation with ethylene glycol is a proposition for
Nicodem to analyze and I happily defer these theoreticals
involving such ring calculus and geometry to him 
and/or to his modeling program chemdraw/chemguess
Maybe this is another PATR " too good to be true " misprint reference ......maybe not .
It would be very interesting to be able to so easily condense the entire assortment of polyhydric alcohols
with the common but so far nearly useless para moth crystals , to form condensates so thirsty for nitro and
nitroester groups  ......and not have to hassle with ......and not have to hassle with
first making dinitrochlorobenzene .
Mem Poudres is mentioned as a reference in the limited
PATR article . This possibility concerning the reported condensation involving para is definitely worth researching , so anyone turning up anything
which clarifies this please speak up .
I have seen some reactions where the chlorine on a ring does pop free easily ....and hoped perhaps this could be
" one of those " times . But if I had to bet , I'd say
it's another PATR misprint and agree with Axt .
Even if there is no getting away from the DNCB ,
as will probably be the case given PATR's talent for
these sort of misprints , the possibility of using DNCB
to form condensates with the polyhydric alcohols still
has interest .
Update :
After a pretty extensive search I can turn up nothing to indicate that PATR is anything but another typo with regards to the reaction of
dichlorobenzene with ethlene glycol .
It appears to be nothing but a misprint as was suspected .
Every reaction found involves the reaction of phenol and ethylene oxide . So it seems pretty clear that the DNCB
or other nitrated chlorinated aromatic compound is still required for any condensation reactions with polyhydric alcohols . Pentaerythritol ,
erythritol , or inositol or sorbitol might form an interesting condensate with DNCB , as a candidate for further nitration . The PE and inositol
condensates would seem to be especially interesting candidates .
[Edited on 24-4-2007 by Rosco Bodine]
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Theres a problem with the need for a solvent to condense the solid polyols with DNCB, any ideas on that?
Tris http://www.sciencemadness.org/talk/viewthread.php?tid=910&am... would be a good candidate for condensation too.
The only readily available isomer of inositol is myoinositol, which seems to only give an explosive goop on nitration so it would be nice to make use
of it. The article i requested provided by Nicodem <a href="http://rapidshare.com/files/27808737/Axt.zip.html">here</a> hoped to provide a
melting point for myoinositol hexanitrate but only makes mention its solid and liquid states.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Axt, N-methylpyrrolidinone aka NMP would be perfect as solvent for coupling of any polyols with DNCB. There are many other polar aprotic solvents that
could be used but as far as I understand this one is pretty much OTC in many countries (USA included). DMSO would also be fine if you can get it.
Alternatively a bit higher boiling ethers like THF, 1,2-dimetoxyethane, dioxane, diglyme, etc. would also be fine. But with ethers you would need NaH
as base while with NMP or DMSO bases like sodium ethoxyde or even KOH might work.
Rosco, I'm not sure if I understood well. Are you asking about the synthesis route as outlined in the attached picture?
If so, then I have to say all would be fine if instead with 1 you could start with p-dibromobenzene and a Goldberg reaction with KOH
/ ethylene glycol catalyzed with 10-20mol% Cu(I) salts or copper powder (reaction A). Heating for 5-10h at 150-160°C with intense
stirring (since p-dibromobenzene is immiscible and not well soluble in the glycol) should give you 2 without much trouble. But with
p-dichlorobenzene (1) this kind of reaction sucks. In some cases chloroaromatics can be used in the Goldberg reaction, but it seldom
works. You could try but you would have to have harsher conditions, like heating up to 200°C and using a cosolvent (diglyme or polyethyleneglycols
would be appropriate). Chances are it would work (though it might also produce traces of the now dreaded chlorinated biphenyls aka PCB's by the
Ullmann coupling, but this can be minimized by using CuCl instead Cu as catalyst).
However, the nitration (reaction B) would probably give a mixture of two regioisomers (3a and 3b)
by the analogy of what happens with p-dimethoxybenzene. Ring trinitration would be hard to achieve without oxidative cleavage (oxidation to quinones).
[Edited on by Nicodem]
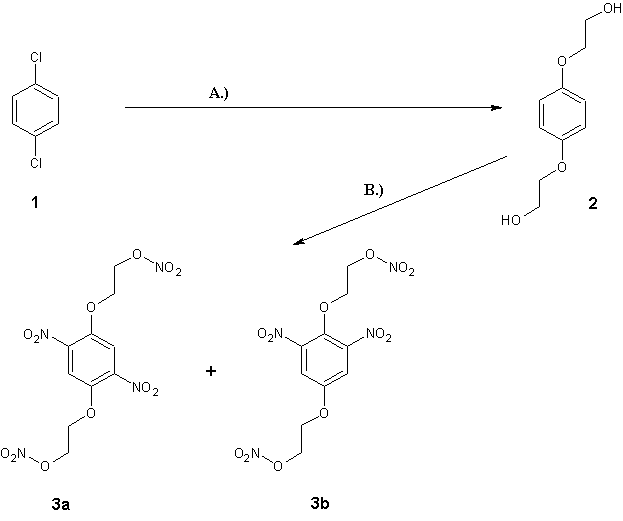
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@Axt
The solvent could be a problem since alcohols could also react with the DNCB . If the reaction was preferential
with the polyhydric alcohol versus a lower alcohol then methanol might work .....but I'm not sure .
For a common solvent I don't know , maybe DMSO ,
or perhaps a ketone .... acetone or MEK .
Regarding the myo-inositol or i-inositol , Crater reported
the hexanitrate as freeflowing crystalline , but on reviewing the stability and sensitivity , and poor temperature stability it is no longer of much
interest .
The stuff is more stable than nitromannitol but is even
more sensitive .....even more than HMTD , and is even flame sensitive , readily DDT's right out in the open when
ignited . Basically it is a crystalline nitroester quasi-primary explosive stronger than tetryl . Touchy stuff to be
much interest in an unknown nitration where its condensate with a nitrated aromatic , would be further nitrated . It seemed like a bright idea at the
time , but the more I think about it the less I like it .
On the other hand the PE condensation with DNCB would seem more plausible . It might be that no special solvent is needed , something like toluene
with a 5%-10% water content , and heated and agitated for awhile as a mixture . An emulsion phase reaction is what I am thinking there .
@ Nicodem
Thanks for looking at this . It was something like the ring
and top chain of 2 that we were looking for , but without
the lower chain dangling there from the second chlorine substitution mirroring the first . Just needed hydrogen there and for the chlorine to go away
as HCl or something ....anything ...just gone , but no such "magic" .
The source of the confusion was another PATR misprint
where the specified dichlorobenzene was a typo for dintrochlorobenzene . And the implication was not a symetrical substitution on both chlorines ,
like you show and which makes better sense , but rather a mono-substitution of that glycol group for only one of the ring chlorines and simple (
unlikely ) hydrogen substitution
for the other to result in phenylethylene glycol ether ,
synonymous with " phenyl cellosolve " or phenoxyethanol , which is a precursor that may be
nitrated to trinitropenoxyethyl nitrate .
It was simply another one of many typographical errors
in the voluminous reference that is PATR , and Axt and I both were scratching our heads and thinking it didn't
sound right . That was confirmed by surveying the
published methods and finding nothing else but a dozen variations on the reaction of phenol with ethylene oxide ,
as the route of synthesis for phenoxyethanol .
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Alcohols, especially MeOH or EtOH would be a very bad choice as solvents for the coupling with DNCB since their pKa is very close to the pKa's of
polyols while they are also more nucleophilic (only t-BuOH might actually work). Isn't NMP sold as a graffiti remover or something like that? This one
should be the best of what can be found OTC. Actually it would be also one of the first choices in a professional lab. However the very first to most
chemists would probably be dry THF and NaH to deprotonate the polyol, but I guess NaH is not particularly abundant outside labs. With NMP you can use
KOH as a base even though it is about 5 times less basic than the deprotonated polyols. All you need to do is assure a bit of excess for the polyol
and drive the deprotonation forward by vacuum distilling about 10% of the solvent while heating the polyol/KOH/NMP mixture. This removes enough formed
water to have a considerable amount of the proper nucleophile. Then you add DNCB and heat to 60-80°C for an hour (or however much it takes according
to TLC). Of course, things are much easier if you use glycerol or some other cheap polyol that can be used in excess. Then you can skip the vacuum and
just use KOH as is.
There is no such one step way from p-dichlorobenzene to 2-phenoxyethanol so there must be some error in that text.
Besides using ethylene oxide, it is also possible to prepare 2-phenoxyethanol by the above described Goldberg aka Ullmann reaction, by using
bromobenzene, which a good amateur chemist should be able to make by himself. Chlorobenzene might be also useful in the benzyne mechanism mediated
nucleophilic aromatic substitution, but that would require heating chlorobenzene, ethylene glycol and KOH in an autoclave at 250-300°C which is a bit
annoying. Alternatively, ethylene oxide can be used prepared in situ by adding 2-chloro or 2-bromoethanol to a solution of phenol in aqueous
NaOH at 40-60°C. This gives a good yield of 2-phenoxyethanol while 2-chloroethanol can be prepared from ethylene glycol.
But are not any such tetranitrated phenoxyethanols inferior by oxygen balance when compared to pentryl? Perhaps it is a bit off topic to discuss them
here in the pentryl thread?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
DMSO would be the most readily available OTC solvent which might work . It is sold in feed stores for a
horse liniment and liniment ingredient for people who
mix their own recipes of muscle rubs . Some people even apply the stuff to themselves .
The OB as compared to pentryl would depend upon how
many nitroesterfiable alcoholic hydroxyls are present on
the condensate of the polyol with the DNCB . In the same way as DNCB condenses with ethylene glycol ,
the next condensate of interest would involve glycerol ,
and then a series of polyols . This opens up many possible candidates for nitration by reactions very similar to what is done for producing pentryl .
The condensation reactions of DNCB to form other useful explosives was mentioned by Axt in this topic , and six
examples were given . This trinitrophenoxyethyl nitrate
which I have submitted as a solidly documented seventh
example is pertinent , and it shows a reaction option
which could possibly add several other similar energetic materials . The condensation of DNCB with TRIS as
Axt mentioned is another possibility also and also more interesting now . What usefulness TRIS may have has already come up in separate discussion ,
but perhaps gains new pertinence here as a possible condensate with DNCB . In that pentryl is derived from the nitration of a condensation product of
DNCB with an aminoalcohol , and Axt has already mentioned lower alcohols also , it seemed pertinent to add the polyols to that list . By bringing
their plural hydroxyls which may be converted to nitroester groups during nitration , it moves towards higher OB and higher energy potential .
The scheme of synthesis and structures are so similar
to pentryl that I believe it is not off topic in this thread
to add this discussion here .
Hey , I know some folks here want to keep me on a short leash , but given insufficient rope how can a man
hang himself , much less tie bow knots around errors in
PATR , and hand them to you as a present  , one , one
more time  Gimme a break . Gimme a break .
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
From paradichlorobenzene, it might be better to use a polyhydric alcohol, even sugars, than ethyleneglycol...or even Tris, as suggested, in order to
increase the number of nitrate ester groups and thus the OB of the final product....
I certainly like the idea of such a condensation.
I was however under the impression that paradichlorobenzene is extremely unreactive - Nicodem, the procedure you cite, is that specific for PDCB or a
generic protocol for chloro aromatics?
[Edited on 25-4-2007 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@Axt ,
In your documentary above , I keep wondering about something there in
" Figure 9: Synthesis of 2,4-Dinitrophenylaminoethanol " .
On the far right side of the equations there you show a byproduct of 2 HCl , and it looks like it should be
just 1 HCl .
For sure in the case where an aminoalcohol is dissolved
in the same non-amino alcohol , the condensation is
preferential for the amino-alcohol . So I suppose what
solvent is useful would definitely be ambiguous ,
to some extent determined by the preferential reactivity of whatever
you may have dissolved in that particular alcohol .
Good news if that proves to be the case , and I am guessing
it probably is that way .
[Edited on 25-4-2007 by Rosco Bodine]
|
|
|
| Pages:
1
2 |
|