| Pages:
1
2 |
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Zhang et al published both of these, in 2016, he tried many different phenols and benzenes, and was half successful (many of them needed cooling to
remain stable), unsure why a salt was unable to be obtained.
In such cutting edge synthesis, i'd remain with his 2017 paper's methods.
Attachment: zhang2017.pdf (623kB)
This file has been downloaded 505 times
Attachment: Zhang 2016.pdf (681kB)
This file has been downloaded 506 times
Sodium Salt from Xu 2017 Nature:
https://www.nature.com/articles/nature23662
| Quote: |
Methods
Safety precautions Caution.
NaN5 and other metal–N5 complexes are, in part, extremely energetic compounds with increased sensitivities towards various stimuli, therefore proper
protective measures (safety glasses, face shield, leather coat, earthen equipment and shoes, Kevlar gloves and ear plugs) should be used. All
compounds should be stored in explosive cases as they can explode spontaneously.
General methods.
All reagents and solvents were purchased from Sigma-Aldrich, Aladdin, and Energy Chemical as analytical grade and were used as received. The
filtration and storage of the intermediate product were performed in a Coolingway DW-86W58 cryopreservation box. A Bruker AVANCE 500 nuclear magnetic
resonance spectrometer operating at 50.69 MHz was used to collect 15N spectral data. DMSO-d6 and CD3OD were employed as the solvent and locking
solvent, respectively. Chemical shifts are given relative to MeNO2 for 15N NMR. High-resolution mass spectra (electrospray ionization) were recorded
on a Waters Q-TOF MicroTM high-resolution mass spectrometer operated in the splitless mode. The samples were dissolved in methanol/H2O (70/30 by
volume), and introduced via a syringe pump at 5 μl min−1. The instrument was run in the negative ion mode with a capillary voltage of 2,500 V. DSC
plots were acquired on a differential scanning calorimeter (Mettler Toledo DSC-1) at a scan rate of 5 °C min−1 in perforated stainless steel
containers under a nitrogen flow of 50 ml min−1. TG analysis was also performed at a heating rate of 5 °C min−1 on a Mettler Toledo TGA/SDTA851e
instrument. X-ray photoelectron spectroscopy (XPS, XPS Microprobe, PHI Quantera II) was performed using Al Kα as a monochromatic radiation source
(hν = 1,486.7 eV) at a power of 240 W (12 kV × 20 mA) at 25 °C. The total pressure in the main vacuum chamber during analysis was typically 5 ×
10−8 Pa. The pass energy was set to 160 eV (energy step 0.5 eV) for recording survey spectra and 20 eV (energy step 0.05 eV) for high-resolution
spectra. The carbon peak at 285.0 eV was used as a reference to correct for charging effects. IR spectra were recorded on a Thermo Nicolet IS10
instrument. Raman spectra were collected using a Horiba-Jobin Yvon Labram HR800 Raman spectrometer with a 514.532 nm Ar+ laser. A 50× objective was
used to focus the laser beam. TG–DSC–MS was performed on a Netzsch STA 449 F3 Jupiter and QMS 403C at a heating rate of 5 °C min−1 under an
argon atmosphere.
[Na(H2O)(N5)]·2H2O (2)
To a magnetically stirred H2O/tetrahydrofuran mixed solution (20 ml/20 ml) containing 4-hydroxy-3,5-dimethylbenzenaminium chloride31 (6 g, 34.58 mmol)
and hydrochloric acid (36%, 3.025 ml, 36.31 mmol), sodium nitrite (2.505 g, 36.31 mmol) in 5 ml water was added dropwise at −3 to 0 °C. After 45
min, 45 ml methanol and 45 ml THF were added and the reaction system was cooled to −38 °C, after which sodium azide (2.360 g, 36.31 mmol) in
methanol/H2O (22 ml, 1/1 by volume) was added dropwise. The resulting mixture was stirred at −38 °C for 1.5 h. The solution was removed by
filtration at −60 °C (in a cryopreservation box), and the filter residue was washed with acetone (10 ml × 4). The off-white solid was collected
and used in the next step without further purification after freeze-drying. A solution of the intermediate product sodium salt of arylpentazole (5.000
g, 21.64 mmol) and ferrous glycinate (8.600 g, 42.16 mmol) in a mixed solution of 100 ml methyl alcohol and 100 ml acetonitrile was stirred at −47
°C. After 30 min, meta-chloroperbenzoic acid (85%, 19.25 g, 94.82 mmol) was added in portions. The reaction mixture was maintained at –43 °C for
24 h. The precipitate was removed by filtration, and the filtrate was evaporated under reduced pressure. The residue was suspended in 200 ml of water,
after which the precipitate was filtered off and washed with 50 ml water. The filtrate was concentrated, and after removing the solvent under vacuum,
the residue was purified by chromatography with ethyl alcohol/ethyl acetate (1/10–1/3 gradient elution). Crude NaN5 hydrate was obtained as an
off-white product (427 mg, 19.56%). Colourless crystals of 2 were obtained by maintaining alcohol (95%) solutions at ambient temperature for several
days. Decomposition point (onset): 111.3 °C; 15N NMR (50.69 MHz, DMSO-d6): δ − 5.7 p.p.m. (s); IR (neat): νmax 3,491, 3,354, 3,293, 2,166, 1,651,
1,246, 1,219 cm−1; Raman (neat): νmax 1,188, 1,120, 1,005, 112 cm−1; HRMS (m/z): [M]− calcd for N5, 70.0154; found, 70.0156; analysis (% calcd,
% found for NaH6N5O3): H (4.08, 4.13), N (47.62, 47.74).
|
[Edited on 6-6-2018 by DubaiAmateurRocketry]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
http://science.sciencemag.org/content/sci/suppl/2017/01/25/3...
Here is the supplementary material for Zhang et al 2017's paper.
The synthesis of the Ammonium-Pentazolate-Hydrate-Chlorate salt is described here:
| Quote: |
An aqueous solution of ferrous bisglycinate (2.65 g, 13 mmol) was added to a solution of
HPP (0.993 g, 5.2 mmol) in a mixture of solvents (160 mL) of acetonitrile and methanol
(v/v, 1/1) and stirred at −45 °C for 30 min. A cold methanol solution of mchloroperbenzoic
acid (4.26 g, 21mmol) was added. The reaction mixture was stirred at
−45 °C for more than 24 h, the insoluble materials were eliminated by filtration. The
2
collected filtrate was evaporated under vacuum to furnish a dark-brown solid. The pure
product could be isolated through a column of silica gel (ethyl acetate/ethanol, 10/1) with
an acceptable yield (19%, based on the number of moles of HPP) of (N5)6(H3O)3(NH4)4Cl
as an air-stable white solid.
|
Note: HPP is n 3,5-dimethyl-4-hydroxyphenylpentazole
Note 2: This synthesis is pretty brutal for amateurs. The below numbers and words are almost painful to read.
m-CPBA
Azides
-45 °C for more than 24 hours.
Filtrate was evaporated under vacuum.
19% Yield.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yikes. 19% yield... The -45 C makes me wonder though, because 0 is easy (ice water bath) and -78 is easy (dry ice/acetone bath) but anything in
between those is kind of weird. Does it really NEED to be at -45, or would it perhaps work just as well at ~-78?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Hmm... they all look like high-yielding products until the last step of the salt.
As for -78... I honestly dont know. Acetonitrile has a freezing point around -50 I think.
Also, I am currently in contact with several vendors, from what I have yeard, azides are getting regulated more heavily, im still confident I can get
it for you though.
I could get the iron bis-glycinate, sodium azide, do you need concentrated HCl, acetonitrile, tetrahydrofuran ?
I can also just obtain the 2,6-Xylenol to make stuff easier.
Edit: Use propane, maybe from a propane tank? Its boiling point is -43. Im pretty sure that'd be fine. Venting large amounts of propane might not be
easy, but it could be done.
[Edited on 7-6-2018 by DubaiAmateurRocketry]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
CaCl2*6H2O and ice is said to reach -55 C, so that may be an option, the dihydrate is cheaply available as dessicant. The required 24 hours may be a
stretch though.
[Edited on 7-6-2018 by nitro-genes]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
the 24 hours is a indeed a stretch, i wonder if it really... needs 24 hours, ill go to the literature and come back and edit this comment.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DubaiAmateurRocketry  | I could get the iron bis-glycinate, sodium azide, do you need concentrated HCl, acetonitrile, tetrahydrofuran ?
I can also just obtain the 2,6-Xylenol to make stuff easier. |
Thanks, though I don't need HCl, acetonitrile,
or THF. I don't want the 2,6-xylenol either because as I explained upthread, the route from that is a lot worse than the route from acetaminophen.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
If anyone needs NaN3 send me a pm, I have plenty.
Edit; I'm only offering to people seriously wanting to give pentazole a try, so basically anyone who posted above or who can give me a believable
story why they didn't post before.
[Edited on 7-6-2018 by Tsjerk]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Quote: Originally posted by nitro-genes  | CaCl2*6H2O and ice is said to reach -55 C, so that may be an option, the dihydrate is cheaply available as dessicant. The required 24 hours may be a
stretch though.
[Edited on 7-6-2018 by nitro-genes] |
The problem is that you still need dry ice to get to that temperature. In my experience, ordinary freezers can get liquids down to about -25 C. If
you're getting dry ice, then you might as well just set the temperature using ethanol/water ratios. 24 hours is still always going to be a stretch
though
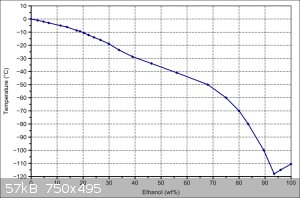
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by Tsjerk  | If anyone needs NaN3 send me a pm, I have plenty.
Edit; I'm only offering to people seriously wanting to give pentazole a try, so basically anyone who posted above or who can give me a believable
story why they didn't post before.
[Edited on 7-6-2018 by Tsjerk] |
Tsjerk, I think texium might be trying to obtain some, also im not sure if your U2U is bugged.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Also on a chinese amateur energetic forum that I follow, a person has claimed to have synthesized (as described in Zhang 2017 et al) pentazolate
salts, and he will be providing some of the information (although not all).
If he does release pictures / procedures i will post it here.
|
|
|
jepa
Harmless

Posts: 1
Registered: 8-6-2018
Member Is Offline
|
|
what are the theoretical conditions that oxides on pentazole can form?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Great question, but first, Welcome to the forum of energetic materials!
There has been speculations that pentazolate-N-oxide can form, or even have 2 N-oxides !! However their stability lacks computational studies, and the
so-far theoretical synthesis I would assume is.
Ozone might just do the trick, but whats tricky is that whether if we need to do it on a benzene-pentazole compound, since ozone will likely produce
benzoquinones instead ? I am not sure if ozone oxidation of pentazolate salts will work.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Some more information regarding Pentazole-N-oxide.
:
Attempted synthesis from DTIC 417664:
http://www.dtic.mil/dtic/tr/fulltext/u2/a417664.pdf
| Quote: |
Cycloadditions of diazenium diolates and other two-nitrogen species with trimethylsilyl
azide in an effort to synthesize pentazole-N-oxides were not successful; catalysts and conditions
will be modified extensively in the continuation of this effort
|
Attempted synthesis of 5-benzyl-pentazole-1-N-oxide:
Attempted Acid-Catalyzed Cyclization of N-Nitroso-N-Methoxy Benzylamine with Trimethylsilyl Azide Under Acid Catalysts to Give 5-Benzyl Pentazole-1
-Oxide 15
Attempt to Effect Acid Catalyzed Cycloaddition of Phenyl Diazenium Diolate to Azidotrimethylsilane Under Acid Catalysts to Give
5-Phenyl-Pentazole-1-Oxide 16
Figure 17. Silylation of dimethyl nitrosamine to give dimethyl silyloxy diazenium triflate.
Figure 19. Pentazole N-oxide synthesis from cycloaddition of azide to diazenium diolate.
Some computational papers:
http://pubs.rsc.org/en/content/articlelanding/2013/nj/c2nj40...
| Quote: |
Starting from pentazolate 1, the synthesis of nitro- and azidopentazole-N-oxide
5–8 can be envisioned by introducing first
either the oxygen atom or the nitro/azido group (Scheme 1).
We have explored computationally the reaction paths for the
synthesis of N- and N,N0
-oxide pentazole-based derivatives. The
mechanism for the oxidation by ozone has been determined
and the synthesis of some of the mono- and di-oxidized
derivatives studied here seems to be realistic, with a regioselectivity
in favor of the b-isomer for the mono-oxidized products. Nitration
can be achieved with NO2
+
BF4
, whereas azide group addition
through electrochemical pathways is not thermodynamically
viable. From pentazolate, the most accessible target seems to be
6. We recommend a synthesis in two steps from 1, with oxidation
by ozone followed by nitration. The oxidation of phenylpentazole
15 prior to N–CPh bond breaking and nitration can also be
envisaged.
|
http://www.hzyxb.cn/oa/DArticle.aspx?type=view&id=201112...
| Quote: |
N5O- 的
分解能垒为12.6kJ/mol,是 N-
5 分 解 能 垒 为1200 倍[20],预测其甚 至 可 以 在 室 温 下 稳 定 存 在
|
Translation:
N5O- has a decomposition barrier 1200 that higher of the pentazolate anion, and might even be stable at room temperature.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
A stupid suggestion probably, could any nitrene mediated ring contraction reactions of azido derivatives be made to work on high nitrogen
heterocycles, e.g. 5-azidotetrazole and known N-oxides?
[Edited on 12-6-2018 by nitro-genes]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Screening benzylpentazoles for replacing PhN5 as cyclo-N5 − precursor by theoretical calculation
https://link.springer.com/article/10.1007/s11224-017-1026-8
(NaN5)5[(CH6N3)N5](N5)
(NaN5)2(C2H4N4)
https://link.springer.com/content/pdf/10.1007/s40843-018-926...
- Not pentazolate, but this is ... polynitrogen in ambient conditions..
Cubic gauche polymeric nitrogen under ambient conditions
https://www.nature.com/articles/s41467-017-01083-5
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Interesting paper:
Synthesis and Characterization of Pentazole Anion in Methanol
https://caod.oriprobe.com/articles/50959132/Synthesis_and_Ch...
https://www.researchgate.net/search.Search.html?type=publica...
|
|
|
| Pages:
1
2 |