| Pages:
1
2 |
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
New Nitrogen Era? Pentazolate (N5 anion) Energetic Salts Synthesized With Low Impact Sensitivity
Lets go back a little bit.
2017:
First "real" synthesis that's not just an observation.
http://science.sciencemag.org/content/355/6323/374
and as usual, a lot of "comments" and critics
http://science.sciencemag.org/content/359/6381/eaao3672
and then the responses, but as they fought on if N5- really existed...
http://science.sciencemag.org/content/359/6381/aas8953
Others made it too! Here late 2017, we have some studies using highpressure to synthesize N5.
https://pubs.acs.org/doi/10.1021/acs.chemmater.6b04538
And then metal-complexes of the N5..
https://onlinelibrary.wiley.com/doi/abs/10.1002/asia.2018004...
and caged structures.. like Na20N60
https://onlinelibrary.wiley.com/doi/abs/10.1002/ange.2017106...

Hmm.. Just wow.
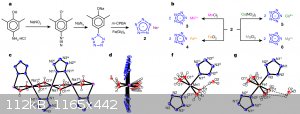
And finally, pentazolate anion as energetic materials! Not the strongest yet, but moderate sensitivity (15J) and around ~8000 m/s VoD
[M(H2O)4(N5)2]·4H2O (M = Mn, Fe and Co) and [Mg(H2O)6(N5)2]·4H2O
https://www.nature.com/articles/nature23662
Energetic Materials of Pentazolate:
https://onlinelibrary.wiley.com/doi/abs/10.1002/asia.2018001...
Cesium Pentazolate:
https://aip.scitation.org/doi/abs/10.1063/1.4971510
[Ag(NH3)2]+[Ag3(N5)4]ˉ
https://www.nature.com/articles/s41467-018-03678-y
(NaN5)5[(CH6N3)N5](N5)3- and (NaN5)2(C2H4N4)
http://engine.scichina.com/publisher/scp/journal/SCMs/doi/10...
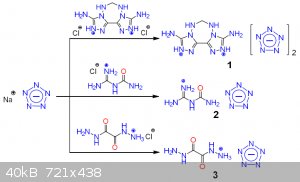
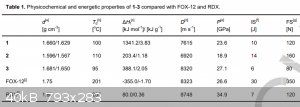
And obviously, what about pentazenium pentazolate? Also, waiting for klapotke on his first pentazolate paper 
[Edited on 2-6-2018 by DubaiAmateurRocketry]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Also, similar to oxides on tetrazole, what are the theoretical conditions that oxides on pentazole can form?
The hydroxylammonium, guanidine, polyaminoguanidines, polyaminotetrazoles, azodicarbonamide, carbazide salts of pentazolate might also exist with some
stability.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
SciMad, let's do pentazoles
Lets take on the pentazole. Sciencemadness has previously made the magical world of the tetrazole a fairly easy task for the average home chemist, so
perhaps we could do the same to the pentazole??
Once we're at the pentazole anion, there's a world of cutting edge chemistry waiting. People took that anion and mixed it with cobalt, iron, silver
salts, and got into Nature! Probably the most prestigious journal in the world. I'm not saying it was easy, but never have I been so excited to try
some cutting edge chemistry. And what's next, nitrogen sandwich compounds? Or just half sandwich compounds? Brand new organo-nitrogen chemistry, it's
all possible.
Pictured is the reaction scheme we somehow need to achieve. Sure, everything needs to be cold, but that's a hurdle we can cross later. First up, comes
the issue of the starting molecule.
2,6-Dimethyl-4-amino-phenol. There's 2 possibilities: either the two methyl 'blocking' groups are a complete necessity, or they're not. Lets explore
the first option first:
That molecule isn't too common. Adding methyl/alkene groups to a benzene ring? Trash. Adding a phenol group? Shit. Adding an amine group? Well that's
not too hard. If you had 2,6-dimethylphenol (from now on called '2,6-xylenol'), a mild nitration followed by a reduction, easy. So is there a source
of 2,6-xylenol? I can't seem to think of an easily available one.
Another option is the plastic Poly(p-phenylene oxide) (PPO). Not all that common but i'm sure everyone would be able to find some around the house. If
hydrolysis is performed however, will it break into 2,6-xylenol, or will it break into 3,5-xylenol? Or a bit of both??
The other option is the the methyl groups aren't strictly necessary. In that case one could use p-amino-phenol, which is only one step away from
paracetamol! But yeah, those methyl groups are likely there for a reason.
Thoughts Scimad?
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I am guessing those methyl groups are there to add electrons to the aromatic ring because that seems consistent with the phenolate so maybe another
deprotonated hydroxyl group or two could replace it. If that is the case then dichloronitrobenzene seems like the easiest way to start.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I looked a bit into p-amino-phenol before and too bad but it is very sensitive to oxidative polymerization (becomes black crap). So unless you can
work under anoxic conditions it will make your life unpleasant (the m-amino-phenol is a lot easier to work with, but not useful here).
I think the poly(p-phenylene oxide) monomer would be a way to get your molecule. I think decomposition of the polymer will give you a lot of crap, as
the polymer is not an ester. Also the plastic is not used in its pure form.
maybe get a sample from a manufacturer?
Ah, I found this:
| Quote: |
The thermal degradation of polyphenylenes and poly(phenylene oxides) was studied under vacuum at temperatures between 350 and 620°C. The volatile and
solid degradation products were analyzed by mass spectroscopy, infrared spectroscopy, and elemental analysis. Overall mechanisms for the thermal
breakdown have been proposed. Polyphenylene decomposes to form polymer carbon, while hydrogen is the major volatile product. Some ring breakdown
occurs with evolution of methane. |
I don't see how hydrolysis could help here.
Edit: as this is quite cutting edge stuff I would stick to the published routes, not trying to substitute/ghetto anything.
Apparently it is a food additive....
http://apps.who.int/food-additives-contaminants-jecfa-databa...
[Edited on 4-6-2018 by Tsjerk]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I agree, I don't really want to substitute anything. It is even dangerous too, it's a lot of nitrogens to herd into a small paddock.
But if we can never getting the starting material, we can never start. The 2,6-xylenol is avaliable on eBay, 30g for $100 so there's always that
option to fall back on, but maybe there is something more inventive?
Good reference about the PPO. The hydrolysis of PET proceeded so nicely but you're right, I'm confusing esters with ethers, it's a lot nice to break
an ester than an ether. It doesn't look like a viable option at all.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Ah, if you can get 2,6-xylenol I would start with that. I don't see any problems nitrating and reducing that. The meta position is de-activated, and
nitrating the para will de-activate it even further.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It should be possible to synthesize 4-amino-2,6-dimethylphenol by acetylating 4-aminophenol and then running a double Friedel-Crafts alkylation with
methyl iodide followed by deprotection of the amine
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
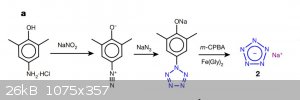
Heres another pentazole synthesis chart - I hope this is more clear.
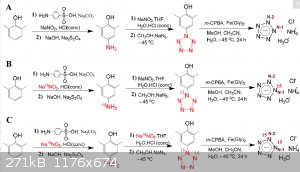
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Also good for treating headaches!
Seriously though, if that FC alkylation works then this is the way. If not, maybe you can pull off a double Mannich reaction followed by reduction.
Also, you could possibly replace those two methyl groups by methoxy groups if you start with syringol and just nitrate/reduce. The nitration would
have to be somehow mild, or you could try a nitrosation.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Duh-doi!
Of course there's no need for starting with p-aminophenol and acetylating it when paracetamol is OTC and already the main source for p-aminophenol for
amateurs! That makes the route even more easy and OTC since it eliminates the need for acetic anhydride.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Has anyone considered propofol as a starting material. There must be some online vendors supplying this material although I doubt it will be
technically legal to import APIs.
Perhaps the tert butyl derivative is more suitable? https://en.m.wikipedia.org/wiki/2,6-Di-tert-butylphenol
Could it be accessible from tert butanol and phenol?
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I wouldn't want to work with propofol due to the legal implications. And even if you could get it with a prescription, it would probably be very
expensive.
[Edited on 6-4-2018 by Texium (zts16)]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Very interesting, but, is all of this too much?
100 grams of 2,6-Dimethyphenol is 20$, from the notoriously costly Sigma Aldrich
https://www.sigmaaldrich.com/catalog/product/aldrich/d175005...
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I suppose you're right, it does seem like a cheap industrial chemical. I can't buy directly from Sigma, and Sigma ships this particular chemical from
the US anyway, but there are members on Scimad we could buy this through?
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
The dimethylation and subsequent de-acylation of acetaminophen really shouldn't be too hard. If there is interest, I would be happy to conduct that
synthesis and send some of the (NMR verified pure) product to anyone interested who would pay the cost of shipping and credit me in their write-ups!
I'd be happy to be part of a collaborative project with you EM'ers.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by Texium (zts16)  | | I would be happy to conduct that synthesis and send some of the (NMR verified pure) product to anyone interested who would pay the cost of shipping
and credit me in their write-ups! |
Synthesis of?
(N5)6(H3O)3(NH4)4Cl ? The pentazolate as described in Zhang et al?
[Edited on 5-6-2018 by DubaiAmateurRocketry]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I guess he is talking about the 4-amino-2,6-dimethyl-phenol
Edit:
In case the amino-phenol seems air sensitive; Couldn't the de-acetylation be done one-pot with the diazotization?
[Edited on 5-6-2018 by Tsjerk]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Hmm, would he really offer to synthesize a phenol compound for $ ? I dont think so, he must be talking about the pentazolate salt (I hope so haha)
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
The amine phenol wont be air sensitive surely... maybe it might want to polymerise but it should be fine in air.
And what Texium is offering is to turn paracetamol into the 2,6-dimethyl-amino-phenol. And then he is offering to donate some, just for the cost of
shipping. He's not trying to make money off of it no, if you want to buy off Sigma go ahead, but I like the cut of his jib.
Personally, I wouldn't be interested in getting some directly from you Texium, but I'd be interested in replicating your results with my own synthetic
hands. In the past I have turned paracetamol into p-DDNP and found that the paracetamol was a lovely starting reagent, it purified and crystallised
well without any hassle and was cheap as it gets.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Tdep is correct- I am offering the 4-amino-2,6-dimethylphenol, which I thought I made clear, but I guess I didn't completely spell that out. I am not
offering to synthesize and ship high explosives! I just figured it could save you a step and give me something useful to do.
Edit: another problem with that compound from Sigma is that it would still require nitration and reduction. The nitration should yield the right
isomer predominantly, but I know from personal experience that reducing nitrophenols with iron is a huge mess. Starting with paracetamol is cleaner,
though you need methyl iodide.
[Edited on 6-5-2018 by Texium (zts16)]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
I dont think the ammonium pentazolate hydrate salt in a solution would be anywhere near explosive. What would a price be for that? I might need for
1-3 grams.
Considering the high impact sensitivity of even the energetic N5 salts see :https://onlinelibrary.wiley.com/doi/abs/10.1002/asia.201800187
I dont think the salt is explosive, you can ship me the sodium pentazolate hydrate version if you want, which is probably less explosive than the
ammonium version.
[Edited on 5-6-2018 by DubaiAmateurRocketry]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DubaiAmateurRocketry  |
I dont think the ammonium pentazolate hydrate salt in a solution would be anywhere near explosive. What would a price be for that? I might need for
1-3 grams.
Considering the high impact sensitivity of even the energetic N5 salts see :https://onlinelibrary.wiley.com/doi/abs/10.1002/asia.201800187
I dont think the salt is explosive, you can ship me the sodium pentazolate hydrate version if you want, which is probably less explosive than the
ammonium version. |
I suppose if the sodium salt is safe in solution, it wouldn't be very hard to make- though
I would need to obtain sodium azide, and determine if I have access to a suitable substitute for mCPBA, which I have looked for in the past but found
to be virtually unobtainable.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
m-CPBA is on aldrich for cheap.
I can financially aid you.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Being able to order from Aldrich is the problem here, friend, not the expense!
Edit: also, on second thought, this is definitely not what I'd consider cheap for a workhorse reagent that is used stoichiometrically. Especially since it's no greater than 77%,
with the remainder being CBA and water...
[Edited on 6-6-2018 by Texium (zts16)]
|
|
|
| Pages:
1
2 |