| Pages:
1
2 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by DraconicAcid  | In organic chemistry, that generally means:
A carbon will have four bonds.
A nitrogen will have three bonds and a lone pair.
An oxygen will have two bonds and two lone pairs.
A halogen will have one bond and three lone pairs.
A hydrogen will have one bond. Period.
In organic chemistry, that will cover about 95% of all molecules. if you add "And sometimes, four bonds and a positive charge" to N, and "and
sometimes one bond, three lone pairs and a negative charge" to O, that covers 99.5% of all organic molecules. |
Hand written into the 'useful stuff' book.
Now all i got to do is properly get my head around the 'lone pair' thing.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Don't do acid while you're learning chemistry. It'll make more sense without it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Dunno, i am starting to think mushrooms might be a good idea for Organic chemistry  . .
Yes i have written it down in my book as well, snippets like that are really useful.
Found the Citric acid to Acetone Synth, well not exactly but the step before Acetone.
It looks like 20% free SO3 is needed, but i dont really see why. I have also read the threads on it and again yields seem low without the SO3.
I might try it at some point with 98% H2SO4 and anhydrous citric acid, but kind of going off the idea.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
If he was still around, Shulgin would argue otherwise, maybe whilst gently prodding the air near your elbow and giggling.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Quote: Originally posted by aga  | Quote: Originally posted by DraconicAcid  | In organic chemistry, that generally means:
A carbon will have four bonds.
A nitrogen will have three bonds and a lone pair.
An oxygen will have two bonds and two lone pairs.
A halogen will have one bond and three lone pairs.
A hydrogen will have one bond. Period.
In organic chemistry, that will cover about 95% of all molecules. if you add "And sometimes, four bonds and a positive charge" to N, and "and
sometimes one bond, three lone pairs and a negative charge" to O, that covers 99.5% of all organic molecules. |
Hand written into the 'useful stuff' book.
Now all i got to do is properly get my head around the 'lone pair' thing. |
I think that means shutting the
door ;^)
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not at all.
These are all stepping stones on the Path young chem walker.
Sadly we're aren't all Born with the most advanced knowledge of Chemistry, so it has to acquired by learning, step by step.
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
and that set of rules is a bit hard to use for aromatics in the case of those new to o-chem. every carbon has 4 bonds except when it has 3 and there
is a delocalised cloud that is represented by a circle. or better still it is represented by the vertex of a hexagon.
Beginning construction of periodic table display
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I know i shouldnt wonder off the path, but i couldnt help look at some OChem books.
I prefer the kind of older way of drawing mechanisms etc, they seem simpler which is good for now. On the other hand i am not sure its a good idea to
learn something that seems to be outdated in newer books.
I should stick with inorganic for now.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by diddi  | | and that set of rules is a bit hard to use for aromatics in the case of those new to o-chem. every carbon has 4 bonds except when it has 3 and there
is a delocalised cloud that is represented by a circle. or better still it is represented by the vertex of a hexagon. |
That's still four bonds, it's just that the fourth one is delocalized between two other carbons.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
@DraconicAcid
| Quote: | | Yes, I've seen it used in several textbooks. It's just wrong. The two oxygens are equivalent due to resonance, and writing them as if they were
different is just silly. |
Following your argument the way carboxylates are drawn usually would be wrong everywhere:

But the point is, it's just a representation, otherwise would anybody assume a molecule is made of letters and lines? What's shown depends on the
purpose. In case of the above image, resonance isn't part of the representation. If the purpose is talking about resonance, another representation is
used.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Dare we mention carbenes? Warning to NEMO: this could be a dangerous tangent leading to a multiplicity of other tangents. 
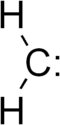
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Magpie  | | Dare we mention carbenes? Warning to NEMO: this could be a dangerous tangent leading to a multiplicity of other tangents. |
Not stable in the least, which is why it's part of the 0.5%....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Does look interesting though..............
Anyone read Organic Chemistry by Donald J Cram and George S Hammond 2nd edition?
Is it any good? I have seen a cheap copy in a charity shop 
EDIT
I should have asked before parting with cash i guess, but there you go impulse buy. Also got 2 sterling silver rings for 25p each  , so silver nitrate on the way nice and cheap. , so silver nitrate on the way nice and cheap.
[Edited on 3-9-2016 by NEMO-Chemistry]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
That's not actually true. If you really want to get technical, even protonated acetic acid will exhibit at least some degree of resonance. Like with
acetate ions, the pi-electrons in acetic acid are also delocalized across the carboxyl group, providing a sort of stabilizing effect on the molecule.
But unlike acetate ions, however, which benefit significantly from that electron delocalization via charge delocalization (the -1 charge isn't
completely localized on any one oxygen atom, but is instead evenly distributed across both), the additional stability provided by resonance in acetic
acid is rather marginal, coming from charge separation instead.
Thus a single acetic acid molecule would most accurately be represented in a fashion similar to how the acetate ion was depicted by Magpie above:
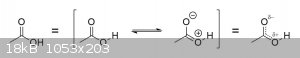
Note the partial-positive charge on the oxygen of the hydroxyl group and the partial-negative charge on the oxygen of the carbonyl group in the
drawing on the right. One of the reasons COOH hydrogens are so acidic and easy to remove is because the oxygen they're connected to is actually
somewhat electron-deficient. This causes the oxygen to not only pull even more electron density away from the hydrogen than it normally would, further
increasing the O-H bond length and decreasing its strength, but it also creates an unfavorable electrostatic repulsion between the two
positively-charged nuclei of oxygen and hydrogen. (the other reason for the unusual acidity is the fact that the hydrogen is only weakly attracted
back to the oxygen atoms upon removal due to the delocalization of the resulting negative charge via resonance)
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have a question on this, but i did to read more before i can ask it in a semi intelligent way.
Organic chemistry is a real struggle to get your head around, extremely interesting but alot of work just trying to get basics to stick.
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
@ NEMO
I think there is an order to learning it all.
1. atomic electronic structures
2. covalent bonding and lewis diagrams
3. structure and nomenclature of organic compounds
4. elementary reactions -- addition, substitution, cracking, esterification, oxidation of alcohols
5. experimental techniques -- distillation, reflux, solvent extraction, acid-base extraction and so forth
6. reaction mechanisms, and more complex reactions
7. manipulating reaction conditions -- equilibria, kinetics, stability of products and side-products
I am up to #5 with the occasional glimpse into #6 having never read a dedicated organic chemistry book -- just general chemistry texts at high-school
level with the occasional bit of undergraduate level reading thrown in.
In all honesty, I think you can get a long way into this stuff with some quite basic knowledge. Structure and nomenclature is just like Lego. But
when it gets complex it ramps up significantly.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
The main problem is i like to experiment, but things can go very wrong when your not sure what your doing.
I have a part time job at the weekends in a cafe, i think i need to save up and get a safer working area.
I dont do anything that risky but a fume hood would go a long way in making things safer. Not just for me but everyone else, I can wear a respirator
but convincing the entire family to sit and watch TV wearing one isnt likely.
I will keep plodding away  . Some very cool reactions in Organic chemistry, so
many ways to make smelly black crud . Some very cool reactions in Organic chemistry, so
many ways to make smelly black crud  . .
[Edited on 5-9-2016 by NEMO-Chemistry]
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Definitely not an expert here but I think you can't go wrong doing a few esters and extractions. You will learn a lot of technique while your theory
knowledge catches up.
And watch everything from chemplayer, doug'skab and nilered.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | Definitely not an expert here but I think you can't go wrong doing a few esters and extractions. You will learn a lot of technique while your theory
knowledge catches up.
And watch everything from chemplayer, doug'skab and nilered. |
I have most the morning in the library doing assorted 'study', perfect time for ear pones and some you tube then   . .
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Aah... That was supposed to be "Doug's Lab". Sorry for the big thumbs on the phone.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
And there was me thinking Doug's skab was a skating boarding channel.
large thumbs and modern phones are like toilet paper filter paper  . .
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
4 vids explaining resonance, first one here
https://www.youtube.com/watch?v=tmeMhbi3Mi8
Might help someone else!
Has a fair few on Org chemistry.
|
|
|
| Pages:
1
2 |