| Pages:
1
2 |
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Why are there two ways to express Acetate?
This might turn out to be a stupid question, so sorry in advance if the answer is obvious.
I have seen Acetate expressed as an equation (especially in books) in two different ways.
For example.
CH3COO-
And
C2H3O2-
Now both ways say exactly the same thing regarding the number of elements etc, but what i dont understand is why have and use two ways of expressing
it?
The only thing i could come up with was, one shows how it splits up (cant think of the correct term!!). While the other is just an overall formula.
I havnt explained that very well sorry, so my question is what does one show/tell you about it, that the other one dosnt?
Or is it just a simple one way was the old way and now its done like this kind of thing?
Thanks and sorry if its a dopey question but i cant find an explanation in the books.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Same reason you can express pentane at C5H12 or CH3CH2CH2CH2CH3. One is simpler, the other gives more information (i.e., shows how the molecule is
put together).
However, I would never write acetate as CH3COO(-), as that suggests that the two oxygens are different in some way. They aren't. CH3CO2(-) is more
accurate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
There is actually a third way of expressing an acetyl group, as "Ac" (OAc or AcO when referring to acetic acid), which is frequently used in organic
chemistry.
Example: Methyl acetate = MeOAc, acetic acid = AcOH or HOAc, 1-hydroxy-2-propanone can be "acetylmethanol" instead.
Basic notation like C2H3O2- doesn't give any indication to the structure or the functional group producing
the charge. Writing the structural formula, or CH3COO-, at least indicates a methyl, a carbonyl, and a deprotonated hydroxyl are
present.
|
|
|
Fulmen
International Hazard
    
Posts: 1717
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Quote: Originally posted by NEMO-Chemistry  |
The only thing i could come up with was, one shows how it splits up (cant think of the correct term!!). While the other is just an overall formula.
|
That's basically it. For something like complete combustion you don't need to know the structure, the net composition is enough. In other reactions
the structure and/or functional groups can be extremely important, not showing these obscures the actual reaction.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
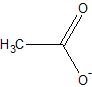
Nothing really beats:
 
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Same reason you can express pentane at C5H12 or CH3CH2CH2CH2CH3. One is simpler, the other gives more information (i.e., shows how the molecule is
put together).
However, I would never write acetate as CH3COO(-), as that suggests that the two oxygens are different in some way. They aren't. CH3CO2(-) is more
accurate. |
Thanks for all the answers.
The CH3COO(-) is actually from 'principles of general chemistry by M. Silberg, one page ref is page 55 of the book, unsure page num of pdf.
I am reading it as one of the books to study, but for now i will skip that part until i go from 'general/elemental' Chemistry on to Organic Chemistry.
The answers have been really helpful, i can see why the different methods exist, but its a little tricky knowing when to use.
At the moment the first way i find more helpful as its easy to see whats in it, but later on i can see i will need to understand how the molecule fits
together.
Cheers
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Yes, I've seen it used in several textbooks. It's just wrong. The two oxygens are equivalent due to resonance, and writing them as if they were
different is just silly.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | | Yes, I've seen it used in several textbooks. It's just wrong. The two oxygens are equivalent due to resonance, and writing them as if they were
different is just silly. |
This is where the real value of a forum shines through, its peer reviewed on the spot and even if there is no consensus, you still get a good
overview.
Books on the other hand while great, leave you at the mercy of the Author.
From a few books i have read lately (must stress mostly general chemistry types), seem to write organic equations in the way illustrated above, i
think more specialised Organic chemistry Text books tend to be more careful in what formula is used.
|
|
|
Texium
Administrator
       
Posts: 4583
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DraconicAcid  | | Yes, I've seen it used in several textbooks. It's just wrong. The two oxygens are equivalent due to resonance, and writing them as if they were
different is just silly. |
What a coincidence, just learned that like half an hour ago in my ochem class. I
hadn't ever thought of it that way before, but it makes sense. Especially since acetic acid then does not exhibit resonance, making CH3COOH more
accurate than CH3CO2H.
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I have to admit that I have seen (and used) CH3COO(-) a lot more than CH3CO2(-). I think it is because visually it is similar to CH3COOH and so its
relationship to the acid is more obvious.
While we are at it, I have seen acetate as both Ac- and OAc- depending on whether the Ac refers to an acetyl group or the acetate ion. The difference
probably relates to whether one is working in OC or performing elementary acid-base work.
This link is relevant and presents all of these ambiguities
https://en.wikipedia.org/wiki/Acetic_acid#Nomenclature
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
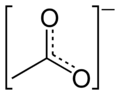
The representation below may be the most accurate but I will not be using it routinely:
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Where is the Carbon meant to sit on that one? I assumed it was the center point but looking at blogfasts it would put it on the end of the bottom left
side?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by NEMO-Chemistry  | | Where is the Carbon meant to sit on that one? I assumed it was the center point but looking at blogfasts it would put it on the end of the bottom left
side? |
There are two carbons atoms in acetate, so both places. Blogfast's drawing depicts one carbon atom, while Magpie's depicts none.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | Quote: Originally posted by NEMO-Chemistry  | | Where is the Carbon meant to sit on that one? I assumed it was the center point but looking at blogfasts it would put it on the end of the bottom left
side? |
There are two carbons atoms in acetate, so both places. Blogfast's drawing depicts one carbon atom, while Magpie's depicts none.
|
Ok thanks, i can see how that fits, no idea how the fuck your supposed to know there is Hydrogen with Magpies one. No wonder organic chemistry gives
so many the shivers..
Edit
The BRACKETS!!!!!
[Edited on 2-9-2016 by NEMO-Chemistry]
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
It is a compact notation. It is efficient but takes a little getting used to.
Hydrogens are not shown and neither are their bonds.
Carbons are not shown but their bonds are. The number of carbon in a chain is indicated by the number of nodes. Thus ethane is indicated by a short
straight line. Propane is two short lines at an angle. Longer chains are shown by a zigzag. (I have no idea how methane is denoted. I guess it
isn't.)
All other elements are shown with bonds indicated as single, double or triple as appropriate.
Resonance is shown as a dotted line to indicate the delocalised electrons.
In general, the most convenient unambiguous form is used in chemical notation. And if isomerism is unimportant in a particular application then an
even simpler form may be used -- for example, C2H7OH might represent both isomers of propanol.
[edit]
The brackets indicate that the whole thing is a species in its own right -- in this case an ion. The negative charge belongs to the unit as a whole
and no attempt is made to show where that negative charge is concentrated. (So even the most thorough notations are a simplification.)
[Edited on 2-9-2016 by j_sum1]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Thanks thats useful info, i am dreading the organic chemistry section on the course!
So many ways to write the same thing, i have books with just lines and others with symbols......It gets really hard to relate one to another even of
the same chemical, i will keep at it though.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OC is crazy complicated IMHO, probably because it is such a vast field, and things keep getting discovered.
Doesn't help when there's more than 1 Name for a substance (and there often is), e.g.
Propan-2-one
Dimethyl ketone
Dimethyl carbonyl
β-Ketopropane
Propanone
2-Propanone
Dimethyl formaldehyde
Pyroacetic spirit
Ketone propane
and more than one way to write the formula :
C3H6O
(CH3)2CO
and even more than 1 way to draw the thing :
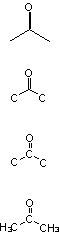
All of these are exactly the same substance, i.e. Acetone.
[Edited on 2-9-2016 by aga]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by NEMO-Chemistry  |
Ok thanks, i can see how that fits, no idea how the fuck your supposed to know there is Hydrogen with Magpies one. No wonder organic chemistry gives
so many the shivers..
|
Whoaa! You can't expect to jump into the middle of a complicated situation and have everything be obvious at first glance! That's what courses in OC
are for - to introduce nomenclature gradually and systematically.
Don't get me wrong: I admire your ambition to learn quickly. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | Quote: Originally posted by NEMO-Chemistry  |
Ok thanks, i can see how that fits, no idea how the fuck your supposed to know there is Hydrogen with Magpies one. No wonder organic chemistry gives
so many the shivers..
|
Whoaa! You can't expect to jump into the middle of a complicated situation and have everything be obvious at first glance! That's what courses in OC
are for - to introduce nomenclature gradually and systematically.
Don't get me wrong: I admire your ambition to learn quickly. 
|
LOL dont blame me! I had no intention of jumping in at the middle, I was systematically going through several general chemistry books (first
mistake).
Right near the beginning of one it had a page that mentioned Acetate, however later on the same page they had written the formula different.
The chapter and book had/has little if anything (at this point) of organic chemistry in it. But having seen two different ways to do it i got curious
as to why.
The book is Martin Spilberg(sp?) principles of general chemistry. I am also reading the Linus Pauling one and the two from the course at school.
My second mistake is having a curiosity that tends to take me on detours!! I havnt posted the latest on things like my adventures with calcium
chloride, mainly for fear of tooting  . .
But a couple of odd things occurred and like a numpty i have gone wandering down avenues that are likely to lead to nowhere! But thats my nature, i
get curious over even the most simple of things.
Its going to lead me to trouble one day for sure, my latest digging around is how to get from Citric acid to Acetone, all because i saw i single
reference to it and have some!
Then i got to wondering about how or if you can get from chloroform to Acetone seeing as you can get from Acetone to Chloroform.
This is why i have taken Chemistry this year, i am hoping it will sort out my random ventures into a more organised and less chaotic style.
So far its just made it worse   
Thanks aga i now know just about every name out there is actually for Acetone 
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by aga  |
and even more than 1 way to draw the thing :
All of these are exactly the same substance, i.e. Acetone.
[Edited on 2-9-2016 by aga] |
Half of those are incorrect.
The top one is a skeletal structure, in which you leave off the carbon atoms, the carbon-hydrogen bonds, and the hydrogen atoms. You can tell where
the carbons are from where the bonds are, and you can tell how many hydrogens have been left off because you know that each carbon has to have four
bonds, and that only the hydrogens are left off.
The bottom one is a condensed structure, in which you don't show the carbon-hydrogen bonds. It's simpler than the full Lewis structure.
The ones in the middle, where you have drawn in the carbon atoms, but not the hydrogens, is not an acceptable way of drawing an organic molecule. If
you do that in any of my courses, I will laugh at you and cast you out of Valhalla.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Why dont books put things like a Carbon has to have 4 bonds! That info alone would of made life simpler.
Thanks for that, and BTW how did you get rid of the 500g of TATP??
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you've learned about Lewis structure, you've surely learned that a carbon atom has to have a full octet. In organic chemistry, that generally
means:
A carbon will have four bonds.
A nitrogen will have three bonds and a lone pair.
An oxygen will have two bonds and two lone pairs.
A halogen will have one bond and three lone pairs.
A hydrogen will have one bond. Period.
In organic chemistry, that will cover about 95% of all molecules. if you add "And sometimes, four bonds and a positive charge" to N, and "and
sometimes one bond, three lone pairs and a negative charge" to O, that covers 99.5% of all organic molecules.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
So you set fire to the 500g then  . .
Thanks for the info, Lewis structure i think is a chapter or two further on. I missed that week of the course as it was week 3, i have only caught
upto week 4 (working backwards).
I know it seems a chaotic approach but its more me spotting something and going off at a tangent.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm not the one that had 500g of TATP, if that was directed towards me.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
No i havnt done acids in details yet. Looks like i got my acids mixed up sorry  . .
|
|
|
| Pages:
1
2 |