| Pages:
1
2
3
4 |
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I was asking about the percolate cell but it is also interesting to know about the plating current density as well.
would it be a problem to have high current density for plating ? I see the PDF uses 5v supply.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1389
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NaClO4
I dont know, what it help us, but here is pretty a new video with clay cup membrane for producing NaClO4.
https://www.youtube.com/watch?v=8ZnYqmbiukI
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Thx Liptakov,
| Quote: |
There are two advantages to this method, you can use graphite as an anode, and you can get 100% conversion due to no cathodic reduction.
|
I think the video explains the idea of PHILOU Zrealone that he expressed in his thread.
I need a little help now to understand this statement "you can get 100% conversion due to no cathodic reduction" what does this mean ?
[Edited on 7-2-2016 by ecos]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1389
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Well, I understand, this: Get 100% change from NaClO3 on NaClO4. It is very important for produce NH4ClO4. Without group ClO3. Or 0,1% ClO3 + 99,9
ClO4. Cathodic reduction (for this type galvanic reaction) is question for PHILOU and others kings of chemistry. LL.
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
It is quite hard, not to say impossible, to obtain pure perchlorate crops by direct electrochemical means and without any further purification. The
resulting cell liquor, even though it mostly contains perchlorate, will still be contaminated by a small amount of residual chlorate. This can be
removed by chemical means though:
http://www.oocities.org/capecanaveral/campus/5361/chlorate/d...
Another means is to purify the perchlorate is by heating to convert the residual chlorate impurities into perchlorate and chloride. The chemical means
probably is more reliable though...
As a matter of fact the thermal conversion of chlorate into perchlorate is actually quite appealing. Considering the simplicity of the conversion
process and the ease with which chlorate can be produced in bulk by the electrochemical method, then it may make sense to omit the hassle of platinum
or GSLD and the electrosynthesis route for perchlorate altogether.
Just produce coupious amounts of chlorate by electrolysis of chloride with MMO anodes and heat it to conversion temperature in a porcelain or
stainless crucible (preferably with PID temp control). For sure the method is less effective in terms of yield, but way cheaper and quite simple:
http://people.ku.edu/~matt915/projects/chloratedecomp.html
https://www.youtube.com/watch?v=EC4OPnvPkLU
The percursor chlorate must be free of organic or metallic contaminants that may cause a firey explosion or decomposition into chloride and oxygen via
catalytic action...
Exact science is a figment of imagination.......
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Good post! I have done the research a few times as well, but never got around to trying the thermal decomposition method. It does seem to have a lot
of advantages over the electrochemical approach. Yield is a bit low, but so what. It is a relatively quick process, with minimal capital investment in
equipment and like you said the chlorate feed is so cheap and easy to make in large quantities.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by Hennig Brand  | | Good post! I have done the research a few times as well, but never got around to trying the thermal decomposition method. It does seem to have a lot
of advantages over the electrochemical approach. Yield is a bit low, but so what. It is a relatively quick process, with minimal capital investment in
equipment and like you said the chlorate feed is so cheap and easy to make in large quantities. |
Thermal method is well known but it is risky. chlorate became very active when heated and any organic existence might end with explosion !
try to avoid it.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Lead Dioxide Electrodeposition Under Ultrasonication : https://www.youtube.com/watch?v=UgYgcymCJ20
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ecos  | Quote: Originally posted by Hennig Brand  | | Good post! I have done the research a few times as well, but never got around to trying the thermal decomposition method. It does seem to have a lot
of advantages over the electrochemical approach. Yield is a bit low, but so what. It is a relatively quick process, with minimal capital investment in
equipment and like you said the chlorate feed is so cheap and easy to make in large quantities. |
Thermal method is well known but it is risky. chlorate became very active when heated and any organic existence might end with explosion !
try to avoid it. |
I am not saying this to be rude, but driving a car can be extremely dangerous too if one doesn't have the necessary skills. Like driving, I am sure
that I could learn to perform this process safely as well.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1389
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
To control dangerous processes, that's great art, that's great beauty. Just as God.
Dr. Liptakov
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I am thinking to electroplate graphite rod with lead dioxide.
I found two different kinds of graphite electrodes, one is black and one is gray.
 
what is the difference between both ?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The grey rods contain graphite and the black one looks like compressed carbon . . . ?
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Vitreous carbon maybe?
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
i think the difference between carbon and graphite electrodes is the crystal structure of the carbon atom.
would electroplating of compressed carbon rod work for chlorate cells ?
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I am ready to build this setup :
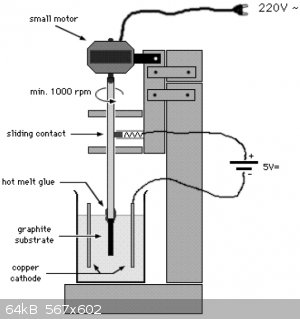
I bought lead , some copper and diluted nitric acid 65%.
I have a fan motor and some beakers.
I think I am ready.
I have a problem now to connect the shaft of the motor with a aluminium rod and to connect the Al rod with the graphite rod.
is there a better way than using glue? I prefer mechanical ways. I wish I find something like a drill chuck that can connect things together with
different dimensions.
any suggestions ?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
This has worked for me in the past for reasonably low torque arrangements:
Plastic tubing (vinyl, polyethylene), from the hardware store comes in all the standard sizes. The outside diameter of one size is the inside diameter
of another. Short lengths of tubing can be used as couplings. If connecting two shafts of different diameters, build up the small shaft with smaller
diameter couplings. If the shafts are standard sizes the tubing will fit snuggly like a glove. If friction isn't enough then drill a hole through all
(shaft and coupling) and put a pin through, or use an adhesive of some sort.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Thanks a lot hennig ,
you mean a tube like this ?? :

I got your idea, this will be between the shaft of the motor and the Al tube.
what about the connection between Al tube and graphite rod?
my problem , I plan to get graphite rods with different diameters (13 mm , 19mm , 23 mm , ..)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
You're welcome. There are at least several different types of tubing available at the better hardware stores around here. Even the more basic stores
usually have vinyl and polyethylene. Also, a tube with the least curvature is best (outside start of a large diameter roll perhaps). With the two
shafts close together in the "coupling" the connection is almost perfectly straight even with tubing with a bit of bend (imbalance of stress from one
side of the tube to the other causing curvature). I think this would work with a graphite shaft as well.
The plastic tubing (coupling) also acts as a torsion shock absorber. Depending on the tubing chosen the coupling can be very elastic. This could
possibly be a good thing when brittle shafts are involved.
[Edited on 27-2-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1389
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Crisis place in the red circle. If the connection is soft, graphite misalignment occurs. You need hard tube against axis displacement.
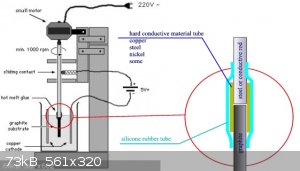
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
How about something like this:
https://www.zoro.com/climax-metal-products-coupling-rigid-st...
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
If it is so soft and so much slack is allowed that it is flopping around when spinning that is a problem of course. However, even relatively stiff
polyethylene, which holds its shape well under most normal conditions, has enough elasticity (is non rigid) so that spikes in torsion force are
greatly reduced (lowered in amplitude and stretched out). Too soft is of course a problem. Not sure it is even needed, it was just a passing thought.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
"I think if someone has lead dioxide powder he can add some epoxy and make stiff anode". I read it somewhere but I don't remember the source.
I found a nice clean technique to make lead oxide : https://www.youtube.com/watch?v=Wzm3N0xPyGU
I think if we add lead to a ball mill and run it for long time. the lead will be atomized and oxidized as well. (I think this is the reason why we add
5% carbon while milling Al to cover it and avoid oxidation)
if this will work we could have lead oxide or dioxide and make anodes easily. I am still investigating this point.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1389
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
As very good idea is clay cap from Philou aparatus. From page 1 this thread. I have questions. Is possible as active surface ( in clay cap garden)
use different lead oxides? Thus only pure Pb3O4 - minium? Has the Minium similarly properties (for galvanic process) as PbO2 ? Or worked Pb3O4 only
with mix in PbO2? 1:1 ? Is posible used Pb3O4 without clay cap aparatus? Will be worked high pressed rod from Pb3O4 ? Thanks,....LL....
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I have a question.
I found some offers on the internet for crucible graphite. some of them are used and with good price.
anybody know if they can work as electrodes?

|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by ecos  | I have a question.
I found some offers on the internet for crucible graphite. some of them are used and with good price.
anybody know if they can work as electrodes? |
Problably, although they might have some additives to make them more suitable for their purpose, I guess.
|
|
|
| Pages:
1
2
3
4 |