| Pages:
1
2
3
4 |
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Graphite Substrate Lead Dioxide anode
Hi All,
I found the cost of Pt anode for perchlorate cells so high and I am searching for other solutions. I like to share my findings since it might help
others.
I think lead dioxide anodes are very good solutions but their cost on internet is still high.
I found three methods to make lead dioxide anode:
1- Burn lead with propane torch to have lead dioxide : https://www.youtube.com/watch?v=eXgzEXjdoWw
I am not sure if this would work or not !
3- electroplating Titanium substrate with lead dioxide on it.
2- electroplating graphite substrate with lead dioxide using electrolysis : https://www.youtube.com/watch?v=D7bpoXKak-4
The second method is called Graphite Substrate Lead Dioxide anode "GSLD". It is discussed in details in the attached PDF. The setup looks like this :
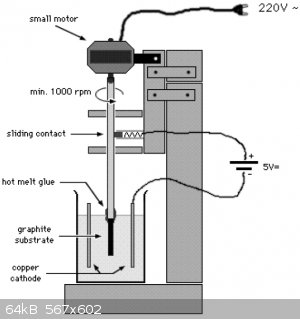
The second video above has implemented the same setup and achieved a very nice anode.
some pictures for professional setup and anodes for motivation :




I think this process doesn't cost much compared to buying the anode from amazon or ebay. I also like the idea of controlling the thickness of the PbO2
layer. I found anodes on internet with 1 millimeters PbO2 layer but it was over $60.
I wonder if I can use magnetic stirrer to circulate the electrolyte instead of rotating the anode. This would make the setup even more simpler.
whats your idea?
More references :
http://www.apcforum.net/forums/blog/swede/index.php?showentr...
http://www.oocities.org/capecanaveral/campus/5361/chlorate/l...
Attachment: HowToMakeSodiumPerchlorate.pdf (211kB)
This file has been downloaded 3711 times
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
The idea of having the electrode attached to stirrer it quite innovative; however, I can see it being a real pain to maintain. Especially with
chlorates and perchlorates, corrosion can be a big problem. It would only take a few days for the motor in the overhead stirrer to burn or short out.
Quote: Originally posted by ecos  |
1- Burn lead with propane torch to have lead dioxide. I am not sure if this would work or not! |
I think this would be very optimistic for this to work effectively unless the Pb is atomized or combusted with an oxidizer.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by Detonationology  | The idea of having the electrode attached to stirrer it quite innovative; however, I can see it being a real pain to maintain. Especially with
chlorates and perchlorates, corrosion can be a big problem. It would only take a few days for the motor in the overhead stirrer to burn or short out.
Quote: Originally posted by ecos  |
1- Burn lead with propane torch to have lead dioxide. I am not sure if this would work or not! |
I think this would be very optimistic for this to work effectively unless the Pb is atomized or combusted with an oxidizer. |
This process is for plating the graphite anode with PbO2. This process takes hours.
I am not alking about spinning the anode in chlorate cell  . .
Fan motor will not burn if you left it run for days 
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Quote: Originally posted by ecos  | Quote: Originally posted by Detonationology  | The idea of having the electrode attached to stirrer it quite innovative; however, I can see it being a real pain to maintain. Especially with
chlorates and perchlorates, corrosion can be a big problem. It would only take a few days for the motor in the overhead stirrer to burn or short out.
Quote: Originally posted by ecos  |
1- Burn lead with propane torch to have lead dioxide. I am not sure if this would work or not! |
I think this would be very optimistic for this to work effectively unless the Pb is atomized or combusted with an oxidizer. |
This process is for plating the graphite anode with PbO2. This process takes hours.
I am not alking about spinning the anode in chlorate cell  . .
Fan motor will not burn if you left it run for days  |
Your info is lucid, I just confused myself with another thread I had open. Sorry for my prior input. Wouldn't copper also precipitate onto the
graphite rod?
[Edited on 11-5-2015 by Detonationology]
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
It's a fairly well tried and trusted technique to minimise pinholing caused by stubborn bubbles adhering to the anode . . .
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
I drew that crappy picture of your setup in the 90's of the previous century. Never expected to see it show up again 15+ years later! Most of the rest
of that pdf is from other sources though.
Copper does plates out, but on the cathode, not on the anode. In fact, the very function of the copper is to prevent lead from being plated out. The
copper preferentially plates out and forms a thin, well-adherent layer on the cathode. If you don't add the copper, a spungy voluminous mess of lead
will plate out on the cathode. This contaminates the cell and causes short circuits.
The original paper I got the method from also included a method to replenish the copper and lead in the plating electrolyte by circulating it through
a system where it first passes through a tank with a mixture of PbO and CuCO3, then through a pH adjustment stage and then back in the cell. I'll see
if I can find a dig up a copy of the paper and post it.
It is not really necessary though if you have enough plating solution and are not plating a very thick layer. The concentration of lead will only drop
very little.
The anodes I got were more shiny than the one in that video. I recall they looked like they were made from obsidian.
[Edited on 5-11-2015 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
"The most important use of lead dioxide is as the cathode of lead acid batteries."
Is the cathode actually plated with lead oxide, or is it a product formed by the reaction of the battery?
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by phlogiston  | I drew that crappy picture of your setup in the 90's of the previous century. Never expected to see it show up again 15+ years later! Most of the rest
of that pdf is from other sources though.
Copper does plates out, but on the cathode, not on the anode. In fact, the very function of the copper is to prevent lead from being plated out. The
copper preferentially plates out and forms a thin, well-adherent layer on the cathode. If you don't add the copper, a spungy voluminous mess of lead
will plate out on the cathode. This contaminates the cell and causes short circuits.
The original paper I got the method from also included a method to replenish the copper and lead in the plating electrolyte by circulating it through
a system where it first passes through a tank with a mixture of PbO and CuCO3, then through a pH adjustment stage and then back in the cell. I'll see
if I can find a dig up a copy of the paper and post it.
It is not really necessary though if you have enough plating solution and are not plating a very thick layer. The concentration of lead will only drop
very little.
The anodes I got were more shiny than the one in that video. I recall they looked like they were made from obsidian.
[Edited on 5-11-2015 by phlogiston] |
It is very interesting to know that you draw that picture  . .
I think this topic is very important due to the high cost of lead or platinum anode
An 10cm x 10cm anode covered with 1mm PbO2 costs $103 : Link
I was amazed when I know about GSLD. I can get carbon rods from batteries and electroplate them. It would be cheaper and I can make thicker layer. It
was very nice to find a video for the whole process.
Didn't you think to circulate the electrolyte using magnetic stirrer ? this would make the setup more simpler.
I think this would avoid the forming of bubbles as well. I am not sure.
I am looking forward for your answer and pictures
[Edited on 5-11-2015 by ecos]
|
|
|
Aurium
Harmless

Posts: 46
Registered: 4-10-2015
Member Is Offline
Mood: Energetic
|
|
This is great. I wish I had known about GSLD earlier!
This makes a homemade electrochemical cell so much more viable.
I wonder if the electrode will resist concentrated sulfuric acid?
Also, how many amps are you putting through a percolate cell?
Instead of hours at 2A maybe one can get the chemistry done in minutes using some 100Amps.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I highly recommend reading a couple of the stickies at the top of the Techno-chemistry section. This thread is what brought me to SM.
https://www.sciencemadness.org/whisper/viewthread.php?tid=50...
I just wish all the links in it still pointed to something.
[Edited on 6-11-2015 by hyfalcon]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Aurium  | This is great. I wish I had known about GSLD earlier!
This makes a homemade electrochemical cell so much more viable.
I wonder if the electrode will resist concentrated sulfuric acid?
Also, how many amps are you putting through a percolate cell?
Instead of hours at 2A maybe one can get the chemistry done in minutes using some 100Amps. |
Depending on quantity, you put 100 amps through your electrolyte and it will boil. You don't want more than 200-400mA per square centimeter dependent
upon electrode material. Any more than that causes undue erosion of your anode.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
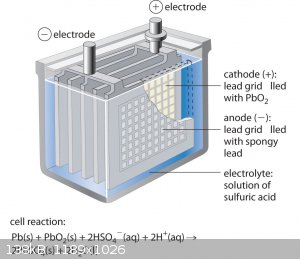
I am thinking to make PbO2 anode using lead dioxide spongy in battery.
I will collect it and then melt it to form a solid electrode then use it for electrolysis.
Even if i have Pb within my PbO2 this won't harm since it would be oxidized from air while melting.
I think this process is toxic. but it should give me high thickness PbO2 electrode.
would this work ? I think it is basic idea
[Edited on 24-12-2015 by ecos]
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
No. PbO<sub>2</sub> decomposes when heated
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Detonationology  | "The most important use of lead dioxide is as the cathode of lead acid batteries."
Is the cathode actually plated with lead oxide, or is it a product formed by the reaction of the battery? |
The first commercial lead-acid battery, by Gaston Planté, consisted of two sheets of pure lead immersed in the acid electrolyte.
The breakthrough that made lead-acid batteries practical for widespread use was Camille Faure's development of the lead dioxide paste electrode. This
consisted of a lead grid lattice, into which a lead oxide paste was pressed, forming a plate.
Here is a page discussing paste electrode manufacture today:
https://www.osha.gov/SLTC/etools/battery_manufacturing/plate...
So the answer is both can be used, but the paste method makes superior batteries.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Then we have PbO2 paste inside car battery.
The key point now is to transfer this paste to a rigid electrode !
phlogiston state that , PbO2 will decompose while heating !
do I need to make electroplating or there is a better technique ?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ecos  | Then we have PbO2 paste inside car battery.
The key point now is to transfer this paste to a rigid electrode !
phlogiston state that , PbO2 will decompose while heating !
do I need to make electroplating or there is a better technique ?
|
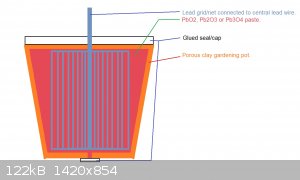
You could also make a porous clay pot (gardening flower pot) to hold the paste and inserted inside the Pb or metallic net rolled in cylinder...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
do you mean something like that :

comment from the website :
| Quote: |
with anodes lasting from hours to months. Pieces of Lead Dioxide were put into a porous plastic bag/wrap. A Graphite rod was placed on top of the
contents of the bag/wrap and the contents + part of the Graphite rod was plated. The bag/wrap used in the picture was mesh material used in the garden
to keep down weeds. |
as you can read, it will not last for long time and not reliable.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ecos  | do you mean something like that :
comment from the website :
| Quote: |
with anodes lasting from hours to months. Pieces of Lead Dioxide were put into a porous plastic bag/wrap. A Graphite rod was placed on top of the
contents of the bag/wrap and the contents + part of the Graphite rod was plated. The bag/wrap used in the picture was mesh material used in the garden
to keep down weeds. |
as you can read, it will not last for long time and not reliable. |
No not like that...a bag will not last long vs a backed clay gardening pot...
I was thinking to something like this:
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I think your idea is based on the very tiny holes in the pot will not allow the PbO2 to be consumed in solution , am i right?
I think the problem here will be the current will be very small due to the small surface area of PbO2. do you agree?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
That is indeed a bit the idea...unsoluble PbO2 paste will remain inside the pot while ions will flow through the porous clay.
The surface will be the inner surface of the clay pot...thus more than your PbO2 plated electrode.
True that the diffusion of the ions through clay may be slightly more limiting than free diffusion in open media...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Ok , It make sense now.
for the lead grid/wire , can it be substituted with metals like SS , Al, Cu ?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ecos  | Ok , It make sense now.
for the lead grid/wire , can it be substituted with metals like SS , Al, Cu ? |
The lead grid I had in mind would be rolled in cylinder, the diagram I posted is a kind of open cut of the system.
The idea is that the PbO2 paste is in contact with Pb so you get the couple PbO2/Pb in the case some side reaction occurs.
The paste will hold intimate with the grid and if it peals off, it simply remains where it is because it can't fall away --> remains in
place...this will circumvent by a simple mechanical way the pealing off effect of Pb/PbO2 electrodes...
In principle this system will remain serviceable for long...as long as the PbO2 remains in the pot --> depends on how log the clay pot will resist
electrochemical cell run conditions and how long it will remain integer vs hot saline solution. Some glazed clay do form micro cracks in
saline/acidic/basic media...here the pot has to be glazedless...because glazed pot will be much less porous to ions...
The use of Al especially (or Cu) is forbidden in the conductive saline media...they would sacrificially corrode first.
Cu may eventually be used out of the mix (inside the Pb wire and over in the air)
Stainless Steel, if it is what you mean by SS, may be tested, but usually it doesn't like chloride, hypochlorite in acidic or basic media...so maybe
just like the Copper --> out of the mix (inside the Pb wire and over in the air) but this may be costly vs Copper pipe and electrical wire...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
I found one of my first anodes made with this method. The coating was a bit thin, but it looks quite shiny and free of pinholes. Never tested this
particular one in an actual cell, but later ones held up quite nicely and produced sufficient perchlorate for my needs (which were quite modest, just
enough for a few small-scale pyrotechnics experiments).

-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
@Phlogiston, Very interesting ! what is the dimension of this anode?
I wish I can make one.
I would like to know the current density you used for your anode.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
The graphite substrate is 8mm diameter and 60mm long.
The current density for plating the PbO2 onto the anode or the current density in the perchlorate cell?
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
| Pages:
1
2
3
4 |