| Pages:
1
..
10
11
12
13
14
..
33 |
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
p-cresol synthesis
nitro-genes, I saw this thread for the synthesis, but it looks like a PITA for something so easily purchased, especially the 0.2% quantity needed. I'm also not sure
what the process is to isolate the para- isomer.
[Edited on 20-8-2014 by roXefeller]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Nice find for the synthesis, I just can't keep up with the massive amount of information that is present on this board. :-) Pure p-cresol is indeed
not easily synthesized, a couple of routes seem feasible. One route is region specific p-chlorination of toluene using KCl and K-peroxosulfate in a
polar solvent, but the problem is that the subsequent elimination-addition reaction with NaOH solution to produce p-cresol requires high temperatures
(350 degC) and occurs via the benzyne intermediate which will result in both ortho and para cresol. Para selectivity in the posted synthesis probably
comes from the fact that treatment of toluene with H2SO4 results in mainly the p-sulfonic acid, in which no water is present during the subsequent
reaction with molten KOH (all reactants probably need to be really dry), maybe that could be the reason why the product mainly consists of p-cresol?
Curious how this reaction mechnism works though.
[Edited on 21-8-2014 by nitro-genes]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
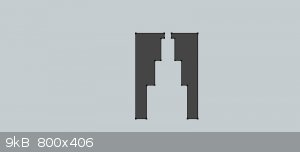
Since DDNP requires such a long run up distance to make the transition from deflagration to detonation (DDT), I thought increasing the column length
of DDNP while keeping the quantity of DDNP to a minimum might be a good option. A stepped internal diameter, reinforced cap to hold the DDNP might
work. Not sure of what the exact dimensions should be. Attached is a sketch of a cross section of a reinforced cap, which illustrates the concept.
This could provide much greater DDNP charge length without increasing the mass of DDNP used nearly as much as if a non-stepped configuration was used.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Perhaps a compound design in the stepped reinforcement capsule would be even more effective: the upper notch containing a different primary with a
short DDT (LA, SADS), driving the DDNP in the lower notch to full detonation without the intermediate deflagration phase.
In that case one actually does not need a notched container...it is pretty tedious to machine on a lathe. Then again, one could fabricate a drill bit
with a notched profile and just bore out the reinforcing capsule from round stock of choice in one go...
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
One may even consider to make a bubble inside the detonator so that you get a shaped charge inside the detonator.
The void caused by the bubble would displace explosive material further so for an equivalent weight and section you would get a longer detonator.
The cavity will create a Munroe effect dard with locally focussed over-pressure, temperature and VOD.
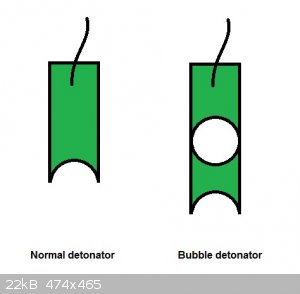
[Edited on 28-8-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Why the stepped design instead of a cone?
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I suppose manufacture of the casing as well as pressing the DDNP is much easier with a stepped design instead of a conical one.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Was kind of frustrated with the benzofuroxan syntesis, so tried something else instead.
This seems interesting, 2,4-dinitro-6-(tetrazeno-I )-phenol from hydrazine and DDNP, couldn't find anything on tetrazeno benzenes or phenols, could it
be that 2-azido 4,6 dinitrophenol is formed instead? The patent calls for a huge excess of hydrazine, I tried on gram scale (1 gram of DDNP in 15 ml
water), freebasing 3 grams of hydrazine sulfate under 15 ml of ethanol. When the ethanolic hydrazine solution was added, exotherm developed, gas
development (fizzing), immediate dark red colour development. After 2 hours at -20, beautifull dark red long needle like crystals form. Yield looks
quantitative. The excess hydrazine does't seem a nessesity at all. What do you guys think? How dangerous would this compound be? (Should have asked in
advance probably  ) )
Attachment: US2728760.pdf (159kB)
This file has been downloaded 713 times
A pea size amount of the crystals were taken out with a plastic spoon and pressed almost dry between paper towels. This stuff is alot faster then DDNP
when dry. Mtach head amount makes a pop, almost like armstrongs mixture (Colour is also almost the same  ) When wet it only smoulders, so I'll leave most of it underwater untill needed. ) When wet it only smoulders, so I'll leave most of it underwater untill needed.
The potassium salt accelerates even faster, 1/8tst of a match head almost makes a crack and is probably on the edge of detonating.
[Edited on 1-5-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There appears to be a typo in the patent shown structure for the potassium compound where the third atom in the side chain is showing as H and should
read instead NH.
Your observation of gas evolution which is probably nitrogen would tend to dispute the structure identified by Kenney in the patent. In the
alternative to a tetrazene linkage side chain, a triazene or hydrazide would be suggested since nitrogen is being lost. Nitrogen is also lost from
DDNP reacting with sodium azide which converts the diazo group to an azido group with the evolution of nitrogen as a byproduct.
See GB412460 attached for the conversion of DDNP by sodium azide to dinitrophenylazide
I am curious what a subsequent diazotization, or a reaction with potassium nitrite instead of potassium nitrate might do to the hydrazine salt of the
hydrazine derivative of DDNP, if the ring diazo group might be converted to an azide. Instead of potassium nitrite, nickel nitrite derived from
calcium nitrite and nickel sulfate might lead to something interesting, since nickel might complex with the hydrazine fragment as a collateral
reaction.
Also I wonder if semicarbazide would possibly work instead of hydrazine.
Attachment: GB412460 Lead Dintrophenylazide.pdf (331kB)
This file has been downloaded 865 times
[Edited on 2-5-2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Yes, also expected the gas was nitrogen, which lead me to believe that maybe the azido compund was formed (although I couldn't see how). I didn't want
to smell it with the excess hydrazine and possibly small amounts of free hydrazoic acid forming. On the other hand, the DDNP was produced from
picramic acid as described in COPAE and used directly after washing. Although it was washed with water, maybe a small amount of nitrous acid remained
which reacted with the hydrazine. Now that I think of it, gas evolution was only evident with the first additions of the ethanolic hydrazine, so this
may suggest that the compound forms indeed as described in the patent.
Interesting stuff though, lots of possibilities creating doublesalts or salts containing oxygen rich cations, etc...:-)
Movie clip of DDNP, the putative tetrazeno,- hydrazine salt and potassium salt will follow.
[Edited on 2-5-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This compound might form a "bridged" neutral binary multiple salt from a neutralization reaction with a divalent metal basic azide or other basic
metal salt, like the basic lead azide or basic lead picrate or basic lead styphnate. Copper and nickel basic salts might do the same but could
possibly also complex with the hydrazine.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Order of ignition:
1. DDNP (Fluffy type, Yellow)
2. Hydrazine tetrazeno salt (Dark red)
3. Copper(I) tetrazeno salt (CopperII nitrate added to the hydrazine salt, Dark green)
4. Potassium tetrazeno salt (Dark orange/browninsh)
The hydrazine salt seemed less violent then when isolated yesterday, curious...Properties of the potassium salt remind me of lead styphnate, exploding
with loud bang, but very little brisance. :-)
[Edited on 2-5-2015 by nitro-genes]
Attachment: DDNP and tetrazeno-salts.avi (3.4MB)
This file has been downloaded 1052 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Interesting. There seems to be a definite jump for the potassium salt. Wonder if the lead salt would be more or less vigorous and how it would
compare with lead styphnate.
You should try a dextrine binder on the potassium salt and let it dry thoroughly before testing it with the glowing splint. See if the higher density
pellet shows a hard detonation or if the higher density slows it down.
A mixture of the potassium salt half and half with DDNP likewise bindered and dried could also be tested to see what the composite will do, if the
bindered potassium salt detonates with more vigor than the loose material.
If the detonation mode is simply a DDT for loose small quantities as is true for lead styphnate, then the binder and accompanying density increase for
a pellet will quench the detonation and the bindered material will simply puff. One of the DDNP patents reported that DDNP will more surely detonate
when bindered, but my tests showed the opposite effect. Strangely however a mix of lead azide and lead styphnate made into a pellet with dextrine
detonates more powerfully than the loose material and the same is true for the azo-clathrate.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Great idea Rosco, one of the ideas I had was indeed to test the brisance of the potassium salt at higher densities snd with confinement against
aluminium plate. Comparing the effects against more dense DDNP and LA. Mixtires of DDNP with the potassium salt also seem interesting, posibility to
overcome the relative long DDT time for pure DDNP. I'll first test with the potassium salt, its extremely low solubiity in water makes that its
synthesis is very straigtforward with quantitative yield. Another thing I would like to try is using hydrazine sulfate/KOH (or possibly K2CO3) to
direcltly produce the tetrazeno salt without freebasing the hydrazine, which is time consuming since it is a very sticky plastic material that has to
be stirred for a long time. How pure was your DDNP? I'm guessing that the dextrin compressed stuff will make DDT, but will try this to be sure.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
DDNP analogue: Styphnamic Acid derivative DDNR
On the preceding page of this thread in this post
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
There was some followup on the DDNP analogue diazo derivative of Styphnamic Acid which has been identified and described in the attached article from
1881, later patented by Von Herz in 1923 in GB207563 for usefulness of the potassium salt as an initiating explosive. This analogue could be termed
DDNR for diazodinitroresorcinol.
It seems likely that there are various reduction methods applicable for the production of Styphnamic Acid similarly as may be done for producing
Picramic Acid. The same factors as apply to the production of Picramic Acid may also hold true for the production of Styphnamic Acid. There is not
much found in the literature about the less well known Styphnamic Acid to provide insight concerning how these analogous reduction schemes may differ
so that any special treatment is needed for the reduction scheme leading to Styphnamic Acid as compared with the better known reduction scheme
preferred for producing Picramic Acid.
According to Von Herz the potassium salt of DDNR showed an initiating ability equal or better than lead azide.
This would seem to fit some of the requirements for a "green initiator". What are the full properties and sensitivity and temperature stability and
chemical stability are not known specifically, but von Herz reported the properties were favorable for use of the material as an initiator.
A later more recent patent US4246052 attached to earlier post here
http://www.sciencemadness.org/talk/viewthread.php?tid=433&am...
provides more information about the properties of DDNR.
Reportedly the magnesium and lithium salts of DDNR are the most soluble and useful for double decomposition precipitations of less soluble metal salts
upon addition of their soluble salts. This may also hold true for the solubility of the styphnamate prior to diazotization, which might be facilitated
by performing the diazotization using magnesium nitrite (exist?), possibly prepared from calcium nitrite and magnesium sulfate, unless precipitation
of the DDNR is necessary to drive the reaction. The progress of the diazotization could be adversely affected if the reaction is solubility driven.
It may be possible that a hydrazine tetrazeno analogue could form from DDNR in similar manner as for DDNP and such analogue may be novel. I haven't
searched to find out if such an analogue has been attempted or identified already. If the reaction does work to produce such an analogue, it would
seem likely the material produced would be energetic, and if it follows the trend observed for the similar hydrazine derivative of DDNP, the hydrazine
tetrazeno derivative of DDNR would be expected to be somewhat more powerful. Depending upon the physical and chemical properties, the hydrazine
tetrazeno derivative of DDNR could possibly be a green energetic more powerful as an initiator than lead azide.
The analogous reaction for producing a hydrazine tetrazeno derivative for the similar analogue from the diazodinitro derivative of pholorglucinol may
likewise occur, if this reaction scheme is general for the entire class of similar compounds.
Attachment: from JCS 1881 Styphnamic Acid related pg1133.pdf (303kB)
This file has been downloaded 716 times
[Edited on 7-5-2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Tested 5 different setups this evening, using 0.1 mm steel tubing, ID 7mm, target, 1.5 mm steel plate (Dent test), 200 Kg/cm2 pressing force
1. 500mg Fluffy DDNP made from picramic acid/HCl at 0 deg C, yellow/brown colour, non-freeflowing (COPAE)
2. 500mg Dense DDNP made from S.Picramate/NaNO2 --> dripping 8% HCl over the course of 1 hour at 30 deg C, dark brown colour, fairly free flowing,
much higher bulk density than COPEA method (Chinese article)
3. 500mg DDNP/Potassium tetrazeno salt (50%:50%)
4. 400mg DDNP + 100 mg Potassium tetrazeno salt pressed on top
5. 500mg Potassium tetrazenosalt
Can make picture of result, but overall no difference in dent depth between the fluffy or dense DDNP, or 400mg DDNP + 100 mg potassium salt. The 50/50
mix and pure potassium tetrazeno salt had about 75% and 50% dent depth compared to pure DDNP, so much less brisant. :-) Like the patent suggested, the
only possible use seems as LS replacement to kick off dex. LA. (DDNP and LA are known to be incompatible, don't know for the tetrazeno salt, won't
test it anyway)
[Edited on 7-5-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It seems counter intuitive that presence of an additional hydroxyl on the ring, such as for DDNR compared with DDNP, would result in a more powerful
explosive than would result from added nitrogen such as the hydrazine derivative of DDNP. But such unexpected results have been observed also for
other energetic compounds where the change in explosive power is minimal or even entirely opposite from what would be expected for different
substituents or modifications. It is mysterious 
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Mysterious it is, amazing to see that the postassium terazeno salt is able to make DDT in <1 mg amounts but performs weakly when compressed, also
really expected more from the 400mg DDNP initiated by 100 mg of the potasssium salt. I would like to test the ability of DDNP and the potassium
tetrazeno salt in initiating more sensitive basecharges as PETN and possibly ETN, DDNP is well known to have much less friction sensitivity than LA.
Tested the potassium salt by rubbing on concrete, expecting it to be fairly firction sensitive owing to its use in primer compositions, but couldn't
make it to go off easily. It also seems reasoably stable, no change after storing for 1 week at room temperature in indirect sunlight and 2 hours at
90 deg C in the oven produced no discolouration.
[Edited on 9-5-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@nitro-genes
There is a nexus between this thread and another thread where you reported nitration of acetaminophen. See the following post in the other thread.
There is a good probability based on the literature descriptions that the dinitration product of paracetamol may be de-acetylated by H2SO4 to provide
isopicramic acid, which may then be diazotized in the same manner as picramic acid, with the resulting product being iso-DDNP which is 4-diazo,
2,6-dinitrophenol, a DDNP isomer which may have properties as an initiator superior to the ordinary DDNP which is 2-diazo, 4,6-dinitrophenol.
http://www.sciencemadness.org/talk/viewthread.php?tid=62204&...
[Edited on 18-5-2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
I think we might have a winner! 
Thanks to Rosco for looking up the references on isopicramic acid, which are not easily found. It seems indeed that the acetaminophen is reasonable
stable in mixed acid nitrations.
Three solutions in separate beakers were prepared:
1. 70 grams of NaOH was dissolved in 200 ml water and put at -20 deg. C.
2. 12 g (2.2 mol equivalent relative to the acetaminophen) of absolutely dry ammonium nitrate was dissolved in 30g 98% sulfuric acid at room
temperature and then put at -20 deg. C
3. Next, to 60 grams of 98% sulfuric acid was added 10 grams of ethanol extracted acetaminophen. This was heated to 70-80 deg. C. for about 15 minutes
to dissolve/sulfonate the acetaminophen. The solution turned a golden orange/brown colour. and then cooled to -20 deg. C.
After all solutions had cooled down to around 0 deg C, the acetaminophen/SA solution was put on an ice-bath (no salt) and the AN/SA mixture was slowly
added using a pipet while stirring. Temperature was remained between 5 and 10 deg. C during the addition. The solution gradually turned from
orange/brown to deep red/orange, to almost back-red, then with the final additions became more of an orange colour again. Judging from the imediate
colour change upon addition of the An/SA, the nitration is very fast, even at this temperature. After 20-30 minutes the addition was complete and the
solution was allowed to warm up to room temperature and kept there for another 30 minutes while stirring. Then the solution was then poured in a
beaker containing 150 ml crushed ice and water.
The stupid thing is that I had removed the NaOH solution from the freezer when the nitration mixture was put at room temperature, it had warmed up
considerably and when added to the ice/nitration mix the exotherm was unmanageable. It heated up to at least 50-60 degrees, I tried adding more ice
water from the icebath, but the beaker was too small. Then I decided to add the remaining NaOH solution anyway producing much heat, after which the
still slightly acidic solution became an almost black-blueish-red colour, probably hydrolysing part of the acetamino goups. It's in the freezer now
and there seems to be a not to shabby amount of blood red crystals at the bottom. Maybe the product obtained is a mix of acetamino-amino compounds and
needs further hydrolysation, but is seems really promising at least. 
It may actually be that if the NaOH neutralization is done at once and at sufficiently high temperatures, the isopicramic acid is produced
immediately, probably at the cost of a slight decrease in yield, due to the (small) remaining amount of HNO3.
I'll try to recrysallize the potassium salt of picramic and isopicramic acid, since the first is a red colour while the latter more brownish according
to one of the papers Rosco posted.
[Edited on 18-5-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Neutralization of the spent nitration mixture (may) not be required. GB24409 describes the product separating simply on quenching the spent nitration
mixture.
The dinitro derivative of paracetamol (reportedly) does separate on dilution of the spent nitration mixture when it is poured over ice. If the
separation does not occur as reported then the neutralization should facilitate the separation.
I am not certain about this aspect, which will depend on the solubility of the dinitrated product of paracetamol, compared with solubility of its
salts. If this reaction follows the scheme for the properties of picramic acid, the free acid will have less solubility than the sodium salt. But
this acetyl derivative of isopicramic acid may differ from the case for picramic acid.
For a neutralization the ammonium salt may be better.
The neutralization of the separated free dinitro compound using a base like sodium or ammonium or potassium, was done for purification and isolation
reasons, or to prepare a more soluble salt in preparation for reduction or diazotization.
For our reaction scheme, it is not needed to even neutralize the material which (may) precipitate from the spent nitration then quenched, cooled and
diluted over ice.
The precipitated material will be filtered and then added to sulfuric acid to be heated to split off by hydrolysis the N-acetyl group from the amino,
leaving the amino.
This mixture will likewise be quenched and diluted and cooled over ice. A cautious neutralization of the deacetylation mixture will be required to
precipitate the isopicramic acid. This is because isopicramic acid is amphoteric, and in the strongly acid deacetylation reaction the isopicramic
acid product actually forms a soluble sulfate. The cautious neutralization of the soluble sulfate, frees the isopicramic acid which is low solubility
and precipitates. The precipitated material should be isopicramic acid. If excess base is added during neutralization, the color will darken
suddenly as the isopicramate salt of the base is an intense color dye. Adding some acid back to the mixture should cause the color to fade again.
The filtered isopicramic acid may then be neutralized in preparation for the diazotization. Or it may not be needed to neutralize, and would depend
on the diazotization method.
My guess would be a soluble isopicramate salt could be useful, possibly the magnesium salt, but the free isopicramic acid alone will likely proceed to
diazotize by sodium nitrite solution with the mixture tending basic being neutralized and even kept slightly acidic by gradual addition of HCl.
[Edited on 18-5-2015 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Rosco, I believe this is the one you were looking for.
Attachment: Thermal Decomposition of Explosives in the Solid Phase. Part I..pdf (1.6MB)
This file has been downloaded 831 times
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yes that is the article, thank you. I deleted the reference request.
Notice toward the end of the article the heat stability of the iso-DDNP is considerably much greater heat stability than for the usual DDNP.
[Edited on 18-5-2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Thanks, I couldnt find the patent you are refering to. Next synth I will try just isolating the product directly from the crushed ice. Anyway,
hydrolysis of the acetamino group is appreantly not that easy as suggested in my previous post. It appears the red crystals were the sodium salt of
the dinitro acetylaminophenol from over neutralizing, it's colour is identical to sodium picramate. When a little acid was added, a yellow/brownish
straw coloured material precipitated immediately, it's colour is identical to DDNP produced from the COPAE method. It was filtered and is at 120
degrees right now in 50% sulfuric acid, smell of acetic acid is very pronounced and solution has gone from yellow brown to brownish red. :-)
[Edited on 18-5-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The picramic acid salts and isopicramic acid salts are both indicator dyes for pH, with the fading of color occurring for the acid freed by low pH,
the color dye effect fading because of the extreme low solubility of the free acid which has a dull and drab color, contrasted with the intense color
of the salt of the acid which is a soluble dye. You don't need a pH indicator for titration of these acids, they are their own built in pH
indicators, because they are pH indicator dyes.
The diazotized product of these acids is likewise very drab in color, dull colored and low solubility, virtually insoluble in water.
Here is the patent GB24409 and the attached article which references the patent see the journal page 1204
Attachment: GB24409 o-nitro-o-amido-p-acetamidophenol.pdf (166kB)
This file has been downloaded 643 times
Attachment: Pages from Journal_Chemical_Society_London pg1203.pdf (380kB)
This file has been downloaded 640 times
[Edited on 18-5-2015 by Rosco Bodine]
|
|
|
| Pages:
1
..
10
11
12
13
14
..
33 |