| Pages:
1
..
8
9
10
11
12
13 |
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
During the experiments, it became clear that CuO can be replaced by CuCl2. In water heating process, this creates Cu(ClO4)2 + NH4Cl. Given presence of
hexamine in solution, perhaps something else as well. Which, according to the result, contributes to brizantion. Other values are the same as in the
experiments described above. So density of output segment is 1.5g/cc per 300mg. The crater depth of 3.1 mm is largest of all investigated variants.
Which is interesting result.

Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Brightelite

Since Brightelite is non-wetting and essentially just an ordinary pyrotechnic compound, it was re-examined. This time to transfer detonation from
basic detonator to booster. Test was successful. Output segment of basic detonator (300mg cavity 6) had same density as booster, i.e. 1.5g/cm3 (1g
cavity 8). VoD at booster output is estimated at 3 - 3.5 km/s. Which is quite a bit for a booster. But, for example, for secondary mixtures based on
TACN, this brisance and power of initiation is sufficient. From which it follows that some more sensitive secondary EMs can be initiated only with
this common pyrotechnic composition. Brightelite thus provides advantage that its brisance may be sufficient for some secondary materials itself.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
What perchlorate do you get when you add copper sulphate to barium perchlorate (in solution) and get a ppt of insoluble barium sulphate with copper
perchlorate in solution. Is it I or II, CuCl04 or Cu(ClO4)2
Barium Perchlorate is Ba(ClO4)2
Yob
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by yobbo II  |
What perchlorate do you get when you add copper sulphate to barium perchlorate (in solution) and get a ppt of insoluble barium sulphate with copper
perchlorate in solution. Is it I or II, CuCl04 or Cu(ClO4)2
Barium Perchlorate is Ba(ClO4)2
Yob |
If you start with copper(II) sulfate then you get Cu(ClO4)2 the copper remains as Cu(II).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Verification experiments were carried out on stability of newly developed substances. Brightelite appears to be stable after one month of
manufacture and cavity filling. And it can initiate ETNs. Lithex is also stable after 2 months from production and filling. Lithex requires a hermetic
seal against moisture after filling. For example, insulation from polyisobutylene rubber (PIB), as in this case.

Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Cool
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 204
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  |
Since Brightelite is non-wetting and essentially just an ordinary pyrotechnic compound, it was re-examined. This time to transfer detonation from
basic detonator to booster. Test was successful. Output segment of basic detonator (300mg cavity 6) had same density as booster, i.e. 1.5g/cm3 (1g
cavity 8). VoD at booster output is estimated at 3 - 3.5 km/s. Which is quite a bit for a booster. But, for example, for secondary mixtures based on
TACN, this brisance and power of initiation is sufficient. From which it follows that some more sensitive secondary EMs can be initiated only with
this common pyrotechnic composition. Brightelite thus provides advantage that its brisance may be sufficient for some secondary materials itself.
|
Is there any preparation instructions for Brightelite? Is it simply mechanically mixed together dry, or is a solvent used with the nitrocellulose
(acetone, etc.) to form a putty and then pushed through a screen to granulate?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Preparation of Brightelite for lazy readers is on page 9 of this thread.
posted on 11-2-2023 at 21:30
[Edited on 12-4-2023 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
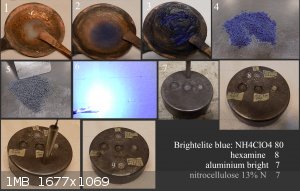
Brightelite Blue
An interesting discovery was made during the research of mixtures of NH4ClO4 (AP) + hexamine. If we heat AP (aqueous solution) in a copper pan, no
copper salt is formed. If we heat hexamine (aqueous solution) in a copper pan, no copper salt is formed. If we heat a mixture of AP + hexamine
(aqueous solution) in a copper pan, a copper salt is formed almost immediately. By experimenting with ratios from 50:50 to 80:8, it was found that AP
only serves as a catalyst. Only the chemical composition of hexamine changes from HMTA to CuHMTA. This has the advantage that Brightelite does not
require mixing with powdered CuO, which is known to promote AP decomposition. Copper is incorporated into the HMTA at the molecular level. Which
supports Brightelite's brisance more than the mere presence of fine powdered CuO.
1) the beginning of the reaction, 2) the course of the reaction 3) the end of the reaction within 10 minutes. Used 300 Celsius air heating with a heat
gun. 4) dried AP+ CuHMTA mixture. 5) dried mixture AP+ CuHMTA + Alu powder + NC 13%N. Grain 1x1mm. 6) demonstration of ignition of this mixture. 7)
Test in steel cavity 8/6mm, output segment 300mg ETN/1.6g/cc. The rest of the cavity filled with Brightelite Blue1.4g/cc. 8) The crater corresponds to
a full detonation of ETN 300mg /1.6g/cc.
9) Output segment 300mg Brightelite Blue compressed to 1.55g/cc. The rest of the cavity is filled with a density of 1.3g/cc provide crater 13x3mm.
Against Brightelite with CuO which provide crater 12.2x3 mm.
Brightelite Blue is no hygroscopic and pH neutral, which is important for present of nitrocellulose at longer storage. Is it basically ordinary
pyrotechical mixture for use as partially blue color flame. Brightelite Blue is not strong enough to initiate secondary EMs, but is strong enough to
initiate ETNs in a solid cavity. The preparation of this substance is almost alchemical. Which adds to the appeal for the scientific madness within
the mixing.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Cool! AP and hexamine form a eutetic when heated as I have mentioned here. With aluminum added it can ddt or act like a primary. But it is sensitive.
Makes sense it picked up the copper. I was able to achieve milligram detonations unconfined. It’s very practical. Feel like I am giving away my
secrets here. But at least it has my name attached to them.
The addition of water is interesting and copper as a catalyst!
AP, hexamine eutetic is like a putty. Should form very high ISP rocket. But could ddt, no core could be used which is also a plus
All being LL if you continue on this path you will find what you want. A mixture that is a NPED.
[Edited on 7-1-2024 by MineMan]
[Edited on 7-1-2024 by MineMan]
[Edited on 7-1-2024 by MineMan]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Thanks, MineMan.....
I also keep an eye on research in the field of Nickel Aminoguanidine Perchlorate. (NAP) I'm too lazy to make aminoguanidine yet. But if I ever make
AG, crazy things will happen...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
Just to let you know I found out that lithium perchlorate is legal where I live and I found a seller. I am ordering 20g of the stuff. Making lithex
will be a real possibility! 
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Thanks, MineMan.....
I also keep an eye on research in the field of Nickel Aminoguanidine Perchlorate. (NAP) I'm too lazy to make aminoguanidine yet. But if I ever make
AG, crazy things will happen... [/ [/
Yah. Hopefully you just buy it so we can see you make new inventions. Why make when you can buy easily.
|
|
|
|
EF2000
Hazard to Others
  
Posts: 153
Registered: 10-5-2023
Location: The Steppes
Member Is Offline
Mood: Taste testing the Tonka fuel
|
|
Quote: Originally posted by Laboratory of Liptakov  |
Brightelite Blue
If we heat a mixture of AP + hexamine (aqueous solution) in a copper pan, a copper salt is formed almost immediately. By experimenting with ratios
from 50:50 to 80:8, it was found that AP only serves as a catalyst. Only the chemical composition of hexamine changes from HMTA to CuHMTA. This has
the advantage that Brightelite does not require mixing with powdered CuO, which is known to promote AP decomposition. Copper is incorporated into the
HMTA at the molecular level. Which supports Brightelite's brisance more than the mere presence of fine powdered CuO.
|
When you do it next time, can you weigh the copper pan before and after the reaction? It would be interesting to see how much of copper goes into
mixture.
Wroom wroom
"The practice of pouring yourself alcohol from a rocket fuel tank is to be strongly condemned encouraged"
-R-1 User's Guide
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
On a laboratory scale for 2 g of the mixture, it will be only hundredths of a gram of Cu. A exact weighing would be quite difficult....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Another breakthrough occurred during the research aimed at initiating ETN as easy as possible. For full initiation of ETN in a solid cavity, it is
possible to use a mixture consisting only of NH4ClO4 and nitrocellulose with a nitrogen content of more than 13%. The tested ratio was AP 80% + NC
20%. Working name Nitrocelite.
0.8 g of AP is crushing in a stainless steel bowl together with 0,2g NC under of acetone level to a homogeneous mass. During mixing, acetone
gradually evaporates at a working temperature of 25 - 30 C. A paste is formed, which can be divided into smaller pieces when wet. These smaller pieces
can then be pushed through a 1x1 mm sieve. It's a bit difficult. Because at 20% NC content, the mixture is difficult to divide into 1x1 mm
agglomerates. It was observed that AP tends to separate from NC during the process. But without negative effect on functionality. Oxygen balance on
CO2 is +18,4. Which is against all rules of pyrotechnics compositions. Despite this, it works. Burning rate on air is relatively low, similarly as
meal BP. Sensitivity on anvil is medium, similarly as safety matches. Output segment ETN 300 mg was pressed on high density cca 30Kg. Follow segment
is 10 mg ETN + 10mg of Nitrocelite pressed handly on 1Kg. Followed insert bridge wire. Followed segment is pure Nitrocelit pressed on handly power 1 -
5 Kg. Others parameters are same as here above. Thus steel cavity 6/8 mm. This assseble provide standard crater 15 - 16 mm, deep 5 - 6 mm. Which is
consistent with full detonation of ETN from other primarily - secondary materials. The mixture is pH neutral and not hygroscopic. From the above, it
is possible to classify Nitrocelite as a material with NPED performance properties. Optionally, the addition of powdered aluminum + 5% is possible.
(or elses catalyzeurs, fuels)
The only disadvantage of Nitrocelite is need to prepare high-quality, well-nitrated nitrocellulose. But for one NPED is nesessary only 200mg of NC
13%N+......
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Just a thought , but couldn’t nitromethane be used as the solvent and help increase the vod of the material?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Quote: Originally posted by pjig  | | Just a thought , but couldn’t nitromethane be used as the solvent and help increase the vod of the material? |
Is it possible but it require exact testing. It was not tryied.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
 It is an amazing solvent for nc or smokeless powders. In this form It has
served as nice vod increase for AN It is an amazing solvent for nc or smokeless powders. In this form It has
served as nice vod increase for AN mix’s with a little Al to increase
sensitivity. mix’s with a little Al to increase
sensitivity.
But in your case I believe it could benefit the mix even in a small amounts . Just my 2cents
[Edited on 18-1-2024 by pjig]
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Another breakthrough occurred during the research aimed at initiating ETN as easy as possible. For full initiation of ETN in a solid cavity, it is
possible to use a mixture consisting only of NH4ClO4 and nitrocellulose with a nitrogen content of more than 13%. The tested ratio was AP 80% + NC
20%. Working name Nitrocelite.
0.8 g of AP is crushing in a stainless steel bowl together with 0,2g NC under of acetone level to a homogeneous mass. During mixing, acetone
gradually evaporates at a working temperature of 25 - 30 C. A paste is formed, which can be divided into smaller pieces when wet. These smaller pieces
can then be pushed through a 1x1 mm sieve. It's a bit difficult. Because at 20% NC content, the mixture is difficult to divide into 1x1 mm
agglomerates. It was observed that AP tends to separate from NC during the process. But without negative effect on functionality. Oxygen balance on
CO2 is +18,4. Which is against all rules of pyrotechnics compositions. Despite this, it works. Burning rate on air is relatively low, similarly as
meal BP. Sensitivity on anvil is medium, similarly as safety matches. Output segment ETN 300 mg was pressed on high density cca 30Kg. Follow segment
is 10 mg ETN + 10mg of Nitrocelite pressed handly on 1Kg. Followed insert bridge wire. Followed segment is pure Nitrocelit pressed on handly power 1 -
5 Kg. Others parameters are same as here above. Thus steel cavity 6/8 mm. This assseble provide standard crater 15 - 16 mm, deep 5 - 6 mm. Which is
consistent with full detonation of ETN from other primarily - secondary materials. The mixture is pH neutral and not hygroscopic. From the above, it
is possible to classify Nitrocelite as a material with NPED performance properties. Optionally, the addition of powdered aluminum + 5% is possible.
(or elses catalyzeurs, fuels)
The only disadvantage of Nitrocelite is need to prepare high-quality, well-nitrated nitrocellulose. But for one NPED is nesessary only 200mg of NC
13%N+......
|
NC has always been underrated as a safe primary. I emailed Axt about this 15 years ago with no response. Great idea. And like Albert said simplest
solution is the best. This is huge, simple, and obvious yet overlooked. Once again, amazing work!!!
NEPDs work best when Oxygen balance is positive, it goes against the rules but my experiments show this is true.
[Edited on 19-1-2024 by MineMan]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Thanks,.....MineMan....
This opens up a wide field of research into different mixtures regardless of the oxygen balance in the future. The oxygen balance can be, for example,
+ 20. For years I stuck to calculations close to zero. But in the construction of NPED, this rule does not apply. I estimate that this finding
(confirmed by your and my experiments) is key information for anyone dealing with initiation compositions.
Clarifications for beginners: Nitrocellulose (by itself) with a nitrogen content of 13% + is not capable of DDT in a fixed cavity from zero to
7300m/s. Which is the table value of VoD for NC in general. And if it ever does, there's no relying on it. Or it detonates at a much lower rate which
is insufficient to initiate ETN.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
For highly nitrated nitrocellulose, commercial nitrocellulose (left) with a 12.4% N content was used as a starting material. For the second stage
above 13%N+, a nitration mixture of 50g H2SO4 96% + 25g KNO3 was used. A sealed jar of reagents was placed in a rotating container at 120 rpm and
nitration was carried out for 90 minutes at 10 degrees Celsius. This was followed by a classic rinse with water and drying. The PLM method was used
for complete neutralization https://www.youtube.com/watch?v=oVZkPWxj5WE&t=50s
The gradually added water contained a 10% NaHCO3 solution. Unfortunately, it was not possible to produce a crystalline NC, but a fluffy NC with short
fibers. See Fig. right......

Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Is there any easy way to find the nitration % of the material? I believe I have the commercial reagent grade 12.5%n . I would like to confirm it’s %
. Can you provide a method you use to test your final nitrated Nc you posted.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Yes. I use incredible exact hammer method for measurement. More sharped sound, more nitrogen in NC...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Lol I like it 
Have you tried that incredible hammer test on the 12.4%n commercial grade NC? Just for a side by side comparison. I’m pretty sure it pops too.
Probably not as energetic or sharp as you describe
|
|
|
| Pages:
1
..
8
9
10
11
12
13 |