| Pages:
1
2 |
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Oh that's because..... um...... hm I have no idea. Why did they use a medium pressure lamp? There's a small peak in the 300-350 range but its
<1/10th the size of the main peak at 250nm.
Maybe you have to always target the first transition in a photochemical reaction? Ground/S0 -> S1 transition, not the S0 -> S2 or whatever? They
must have used a medium pressure lamp for a reason, but I fail to see how the Supp Info figure shows that "300-350nm is a critical region"
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
I also fail to see why one peak should be favored and would love an explanation.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
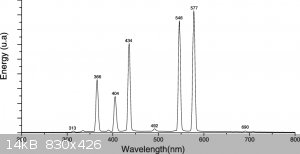
In the article they state their lamp is inefficient for this process, and 300-350nm is the optimum... So I'm leaning towards the 310nm lamp...
Edit: screw it, I just bought the 310nm lamp. If it doesn't work I can always buy the 254nm one, those are only 10 euro and fit in the same socket.
[Edited on 30-6-2020 by Tsjerk]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I just realized they use a pyrex reaction for the photocyclization, which absorps anything below 275 and most below 300nm anyway... not sure what to
make of that. Would that mean lower wavelengths do work but are not considered as they are not transmitted by the pyrex anyway, coming to the
conclusion 300-350nm is the optimum because that is the lowest that can pass. Or did they use the pyrex reactor because 300-350nm is the optimum
anyway?
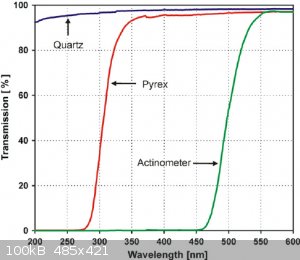
Edit: this is the spectrum of a medium pressure Hg lamp after pyrex filtering the light.
[Edited on 1-7-2020 by Tsjerk]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
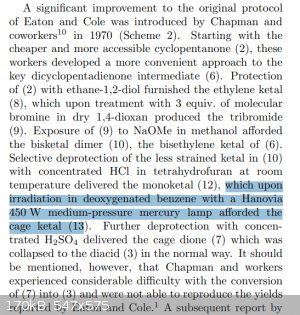
What's also strange, is that the medium pressure lamp seems to originate from this reference, where they did the photo-reaction in... benzene?? A
solvent that would completely absorb all <300 nm light in the first centimetre of the reaction vessel.. completely impenetrable.
So did they use a medium pressure lamp because they had to use benzene for the reaction, which forced them to use ~350nm light? Or did they chose
benzene because the <350nm light doesn't matter? Or was the medium pressure lamp just what they had and everyone just did it too? Does everyone
hate quartz glassware? Is this a conspiracy????
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
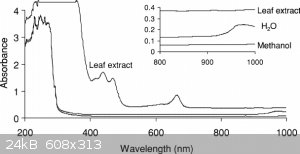
Well, the 85:5 methanol/water solvent system used by Falkiner et al. also absorbs significant amounts of everything <300nm, just as the benzene
does. So I don't think conspiracies theories are needed here as an explanation (bummer), and pyrex is just cheaper than quartz.
[Edited on 1-7-2020 by Tsjerk]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
That's a weird diagram, because I know methanol is transparent to UV down to about 215nm, I've used it as a solvent for UV-Vis. Methanol/water mix
won't absorb the 250nm low pressure mercury emission peak at all. But yes, maybe the critical region really is just where the substance absorbs but
normal glass transmits fine
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The scale on that last graph is logarithmic, so absorption would be around 10% max indeed.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I contacted the corresponding author of the Falkiner article and he told me that 300-350nm is the target. They use Pyrex on purpose because shorter
wavelengths would induce unwanted side reactions. He said the mercury lamp produces about ten percent at the correct wavelengths, so with about 200
watt they can transform 0.25 gram/minute.
[Edited on 1-7-2020 by Tsjerk]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Alright, I guess that's the answer then, good choice on buying the UV-B bulb. Excited to see how you go with it!
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Update: I'm pretty sure I finally got compound 10 (the dibromdicyclopentadiene-1,8-dione ketal)... The bromination and
dehydrobromination were straightforward, just put the reactants together and stir/reflux. After adding water a brownish precipitate forms which settle
to the bottom after some time.
In a separatory funnel I got rid of most of the supernatant, but then trouble started...
The brown sludge is impossible to filter, the stuff is so fine it clogs any filter in minutes. I managed to pull of a large part of the water over
many hours over a high vacuum on a large Buchner filter. I then evaporated much water under vacuum over a 50 degrees water bath. The sludge was then
left to stand for a couple of weeks after which most of the water evaporated.
The last 100 ml of water was pulled of again on a Buchner filter. After an hour the brown (partly black) product was extracted with ethyl acetate by
boiling and hot filtering the mixture. A little black crud was left, but probably only 5-10% of the amount of product.
The solution in ethyl acetate is now evaporating, as in the freezer only water froze out. The solution has an orange color, but I saw some nice
white/colorless crystals in the brine I used pull out the water from the ethyl acetate before leaving it to evaporate.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I've been watching this very cool project with some interest. I'm tempted to buy some adipic acid and follow along.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Cool! I can advice when making the ethylene diketal to run the Dean Stark as slow as you can. Ethylene glycol and toluene also form an azeotrope (110
degrees) which I'm sure screwed up my yield considerably by making me believe the reaction was done.
During the vacuum distillation of the ketal I'm pretty sure I also distilled some glycol as at the end a more viscous liquid came over. I wouldn't be
surprised if my bromination/dehydrobromination would have been a lot less black if my ketal would have been cleaner.
I guesstimate I have about 30 gr of compound 10 now as really nice white crystals. They crystallize from the orange liquid, and there will probably be
an orange crust when totally dry, but I won't try to clean that up as in the article they talk about a beige powder used without recrystallization.
Edit: I started with 40 gr of ketal.
[Edited on 9-8-2020 by Tsjerk]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
So I'm officially very invested in this project, here's my into video outlying the plan: https://youtu.be/ney-qTU3m4I
and my first attempt at making the ketal: https://youtu.be/sBA_UV15k0c
It'll be nothing new to you Tsjerk, or anyone following this thread closely. Looks like i've made a lot of mistakes too... aldol condensation...
ethylene glycol-toluene azeotrope (I hadn't thought of that, that explains what I was seeing in the dean Stark I think!).... but isn't everything so
much easier with hindsight! Hopefully the next attempt will be much more refined.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Very nice video on YouTube Tdep! Good idea to not do the bromination and dehydrobromination in one pot. Your work-up looks a lot easier than my
filtration did. Hopefully this won't happen after you do the dehydrobromination...
I do think the use of less than an equimolar amount of dioxane is what caused the off-gassing, not the use of DCM as a solvent. Bromine doesn't
dissolve in DCM very well, while it forms a complex with dioxane. I think the combination of free bromine and HBr causes the gas to come off. I didn't
notice any gas coming off.
Edit: I once dried sieves in a microwave/oven, thinking I turned on the oven part... Turned out I turned on the microwave part for 20 minutes which
completely fused the sieves, the beaker and the glass plate in the microwave/oven combination. Apparently sieves happily absorb microwaves.
I was noticed by the smoke alarm things weren't going as I intended as the rubber lining below the glass plate was being turned to tar.
[Edited on 27-12-2020 by Tsjerk]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Btw, I performed the de-ketalization. Straight forward process, I performed the reaction on a day the temperature in my lab was about 25 degrees
during the day and didn't drop much at night. Good to know is that the reaction starts off quite exothermic, so cooling was necessary at the start.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Help with NMR spectra
Hey everyone!
So I'm still working on this project, you might have seen my videos on Youtube already but I'll link the series: Cubane Youtube playlist
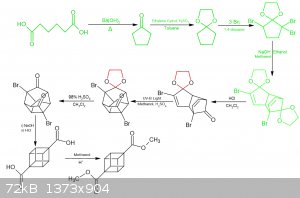
It's been a lot of fun so far, and I'm up to the deprotection/de-ketalization stage. Rather than removing both ketal groups in one step as per the
reference at the start of this thread, I'm intending to deprotect only one side of the molecule. See the reaction scheme. This apparently makes the UV
photochem step cleaner, which is looking like the biggest hurdle of this reaction scheme, so any help there is important. This comes from "CUBANE
DERIVATIVES FOR PROPELLANT APPLICATIONS" (1989).
So I tried the step, ended up with some powder that looked like my starting material after 9 hours of reflux. Recently however, I got the opportunity
to send some samples off for NMR analysis! I sent the diketal starting material (from the end of Episode 7) and some material from the deprotection
reaction pot (End of Episode 8)
It's been a few years since I had to do NMR, and even then I wasn't very good at it.... so sorry for the multiple pictures, but is the following
interpretation correct?
"Using the 1H peak integration, the monoketal reaction mix is 15% diketal and 85% monoketal, with no traces of the fully deprotected product?"
The first 3 images are NMR predictions of the NMR spectra of the diketal, monoketal and fully deprotected molecules. Thanks!
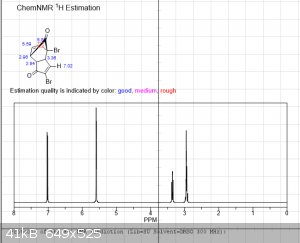 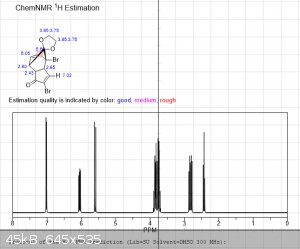 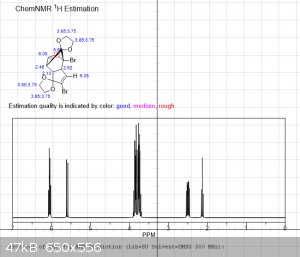 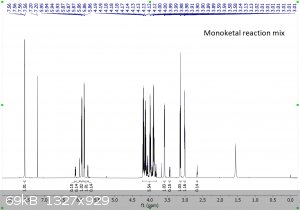 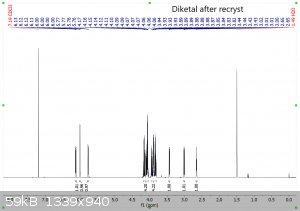 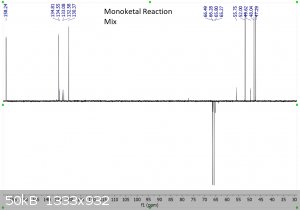 
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Here's the reference that's been a really big help for me. Lots of tips in this about the synthesis of cubane, many of which are in the falkiner2013
paper, but there's a lot more detail. Such as them reporting different solvents they dried which didn't work, and ones that worked okay but not that
well, not just reporting it being done in one solvent and not expanding on it. Cool stuff
Attachment: CUBANE DERIVATIVES FOR PROPELLANT APPLICATIONS 1989.pdf (2.7MB)
This file has been downloaded 353 times
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yeah, so going by intergration it looks like the ratio of diketal : monoketal is roughly 0.15 : 1.03
Which is a molar percentage of 13% diketal. The mass percentage will be a bit higher because of the higher molecular weight.
I wouldn't be sure that there's "no trace" of the fully deprotected product; there are a few very small peaks that could be it. You should try to find
a literature spectrum for the fully deprotected product so you can compare.
edit: also, as you can see, "ChemNMR estimation" is terrible for stuff like this. It's possible to make better estimations using QM software like
Gaussian, but this can be a bit tricky.
edit edit: also I'm a huge fan of your channel and I'm very impressed by this project!
[Edited on 2021-3-7 by Metacelsus]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Thanks mate!
Quote: Originally posted by Metacelsus  |
I wouldn't be sure that there's "no trace" of the fully deprotected product; there are a few very small peaks that could be it.
|
Because I'm not super familiar, what sort of statement could I make about the purity then? Just in a sort of handwave-y way. Less than 5% of other
products? Or it's impossible to say?
Yeah I haven't found one for the fully deprotected one. There's some reference stuff from the 1989 paper for the diketal and monoketal, although it's
a scanned copy so the quality is pretty awful. But there's nothing in there that doesn't match to my spectrum
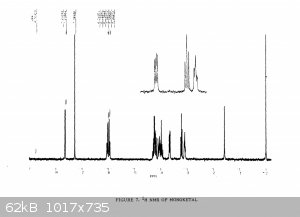 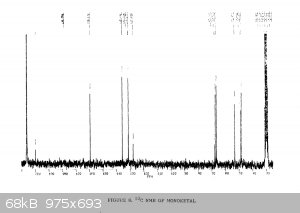
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yeah it's certainly less than 5% fully deprotected product. It looks to me like less than 1%.
Assuming your product is free of inorganic salts, it's almost entirely monoketal and diketal. There is also a bit of water too, but sometimes that's
from the NMR solvent not being dry and not from your product, and I can't tell in this case. I also think I see some DCM around 5.3 ppm.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Thanks a lot. Yeah the reaction was water and DCM, so no surprise that they show up, good spot with the DCM! But very cool. I'm amazed the products
have come out so clean honestly. I've sort of been fumbling along and for the products to be 99%, even with all the possible isomers and impurities is
sensational to see!
|
|
|
| Pages:
1
2 |