| Pages:
1
2 |
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Cubane; Dimethyl Cubane-1,4-dicarboxylate synthesis
I'm far from the full synthesis of dimethyl cubane-1,4-dicarboxylate, but I'm getting somewhere.
Based on this publication: Attachment: falkiner2013.pdf (1.4MB)
This file has been downloaded 750 times
The first step I took was making cyclopentanone ethylene diketal. presuming the product is pure, I did it with a yield of 69%. I'm planning to do a
vacuum distillation before proceeding, so that will go down a bit.
What I did:
To make compound 8 from scheme 5 I bought cyclopentanone and ethylene glycol. As a catalyst for the ketalization I refluxed 25 ml of 96% sulfuric acid
with 100 ml of toluene in a Dean Stark apparatus. I don't know if it is normal for this reaction to turn black, but after one hour of reflux at least
the sulfuric acid layer seems to take up all the black tar (I guess it is tar) and the toluene layer on top becomes quite colorless, it has a bit of a
green color. I stopped the reaction after I separated 2 ml of water in the Dean Stark trap.
I screwed up the above preparation once, I tried to separate the two layers (toluene/sulfuric acid) in clean, but not dry glassware. This will make
the p-toluenesulfonic acid (TsOH) precipitate and clog the separation funnel. The TsOH hydrate is pretty insoluble in my pretended solvent (toluene)
so I didn't try to get it back in solution or to do a laborious isolation of TsOH, which would also introduce a water molecule as it forms a monohydrate. With dry glassware, everything worked out
fine. I just separated the two layers.
50 ml of the above prepared solution of TsOH was diluted with 150 ml of toluene and to this cyclopentanone (30.0 ml, 28.5 gr, 0.34 mol) and ethylene
glycol (19.0 ml, 21.1 gr, 1.05 eq.) was added. This was refluxed for one hour at high heat in a Dean Stark apparatus, after which the calculated 6 ml
of water was trapped by the Dean Stark. When heating was continued droplets of ethylene glycol were captured by the trap (ethylene glycol forms an
azeotrope at 110, against the water azeotrope at 84) which was visually determined as the droplets sank into the water layer.
To be sure the reaction was finished (as I didn't know how much glycol came over with the first 6 ml) the water and ethylene glycol captured were
returned into the boiling flask and the procedure was repeated but with lower heating. This time there was a clear difference in viscosity of the
droplets captured before and after the 6 ml mark.
The reaction was cooled and washed with bicarbonate and brine. The toluene was evaporated under vacuum and the remaining organic layer was dried over
magnesium sulfate. The crude yield was determined to be 30 gr. 69%.
I did screw up during the work-up, I had a suck back of (clean) water during the vacuum evaporation of the toluene, stupid mistake which made me dry
the organics again, so the yield probably could have been higher. Also next time I will consider using worked up TsOH ( the hydrate will be captured
by the Dean Stark anyway...) or something like acidic exchange resin as a catalyst (from a Brita cartridge?). But for a first attempt, not too bad I
would say.
[Edited on 15-2-2020 by Tsjerk]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The next step is the bromination of the diketal with 1,4-dioxane.Br2. I'm looking forward to receive my sodium bromide and sodium bromate, as I wasn't
looking forward too much to distilling bromine. With the bromine/bromate and a bit of acid I can make bromine as a separate layer which will be dried with sulfuric acid.
Apparently this step only works with the dioxane-Br2 intermediate. The solvent doesn't seem very relevant to me... the reaction runs at room
temperature. Only the dioxane/bromine complex seems to be important. I'm thinking about using a co-solvent instead of pure dioxane as the solvent.
The reaction scheme:

[Edited on 15-2-2020 by Tsjerk]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That looks pretty interesting. I have never seen this synthesis, but it looks good, practically a good organic test question. For most cases of
the first step, you could simply start the reaction by mixing the ketone and glycol in toluene and then just add a little sulfuric acid as the
catalyst, I believe. But pre-making toslic acid that way is a neat trick.
The bromination is interesting, it goes only 3/4 of the way, likely due to sterics. Then the dehydrobromination to the monobromo-cylcopentadiene,
which does a Diels-Alder with itself. Good luck with the whole synthesis.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Dr.Bob  | | For most cases of the first step, you could simply start the reaction by mixing the ketone and glycol in toluene and then just add a little sulfuric
acid as the catalyst, I believe. |
I was planning on doing that; but when I added the sulfuric acid to the toluene it turned black in minutes. I don't know if this happens to pure
toluene or that it is due to an impurity that is polymerizing. It does look a lot like tar and the color is captured by the sulfuric acid layer after
prolonged refluxing.
[Edited on 16-2-2020 by Tsjerk]
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
The polymerization is most likely due to (1) impurities, or (2) some sort of interesting polymerization with the ethylene glycol. Is the
toluene/sulfuric acid fairly pure?
If they are, and the black tar still happens, I highly recommend making the tosylic acid first. Chances are EG polymerizes messily in the presence of
sulfuric acid.
The philosophy of one century is the common sense of the next.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Thank you for the reaction! Did you run the tosylic acid preparation yourself with pure toluene? I wonder whether it turns black with pure toluene.
What I did was running this preparation in two steps, first preparing the TsOH catalyst; see if I could capture some water in the trap, and than use
the TsOH solution for the ketalizaition. I thought it would work as an one pot reaction, but it looked so dirty I separated the sulfuric acid (full of
tar) from the toluene before using it. It forms a nice two layer system, and the final toluene layer was only slightly colored.
I guess my toluene contains something that polymerizes, It was dirt cheap...
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
I have, indeed, carried out the tosylic aicd preparation with pure toluene, and no such black tar had formed; the most discoloration was a slightly
gray or a light orange-yellow. I suspect it has to be impurities and/or reaction with EG.
The philosophy of one century is the common sense of the next.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The black tar was separated with the sulfuric acid layer before I added EG and cyclopentanone. After removing the tar and dilution of the TsOH with
fresh toluene the solution was only slightly yellow, as is the diketal now.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I'm really interested in doing this. The pilot scale synthesis paper is fantastic, and I found that before I found the thread, thinking "hey wonder if
anyone from sciencemadness has tried this"... of course they have.
The difficult part of this experiment for me is the need for vacuum distillation. The boiling point of the ketal is what... 150 C? Distilling at
standard pressure might "tar it up" at those temperatures I suppose... or break it apart and start distilling the two fragments... although there's no
water left in the system?
An issue I've been thinking about is 1,4-dioxane production. You do have excess ethylene glycol and a hot, acidic environment. So maybe the vacuum
dist. is to avoid the temperature needed to drive dioxane production?? But then you see in the Pilot-Scale paper, that they use dioxane as the solvent
for the next step... so I guess who cares anyway?
If it's all about keeping the temperature low, could you run a Dean-Stark set-up with Benzene instead of toluene? Boiling point of azeotrope (91%
benzene) is 69 C, and then after the reaction you can distil off the benzene at 80 C, leaving behind just your product, some small amounts of
dioxane.... and you proceed to the bromination without further purification?
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
On second thoughts, you'll have acid and excess ethylene glycol and potentially cyclopentanone and knowing me some tar... so it's probably worth
distilling out the product before doing the bromination, right?
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Sigma says the boiling point of the ketal is 55 °C at 35 mmHg. That vacuum level can be easily reached with an aspirator. This whole synthesis looks
very doable! Any updates Tsjerk?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I indeed vacuum distilled the ketal, of which I have plenty now. The bromination went fine as far as I could tell but I couldn't filter the product,
it was so fine it went straight through my finest glass filter. Gravity filtration also didn't work as the filter was clogged within seconds.
I left the product in an open beaker, the solid at the bottom seems stable, it stays nice and beige, but as soon as the liquid evaporated everything
turned black.
I'm producing a big batch of KBrO3 out of KBr by electrolysis as we speak, so I can make more bromine. I will brominate some more ketal soon and
figure out a way to filter the product.
One problem I'm facing is the source of UV in the 300 - 350 nm range. I have a nice quartz tube I can stick through a Teflon stopper, but looking for
a UV led only got me here. The have leds's in the right range but they are expensive... They have a price list somewhere on the website.
Does anyone know a cheaper source?
Edit: about the tar produced by the toluene/sulfuric acid: I later found that happens with technical toluene and can be prevented by stirring the
toluene with sulfuric acid at room temperature and seperating the acid before doing the Dean Stark.
sulfur in toluene
The yellow ketal nicely cleaned up by vacuum distilling it, indeed leaving black crap behind.
Edit2: I'm thinking about extracting the brominated product with DCM... This would then be dried with MgSO4 and evaporated under vacuum. That way
there is no need for filtration. Also the product seems to be oxygen sensitive, this way not too much air would be around.
[Edited on 25-6-2020 by Tsjerk]
|
|
|
Endo
Hazard to Others
  
Posts: 124
Registered: 5-1-2006
Location: USA
Member Is Offline
Mood: Cold
|
|
I recently purchased some UV LEDs from these guys.
EBAY supplier
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
The wavelength has to be shorter than 350nm, which is frustrating, as most common UV LEDs are 365 nm. There's some deep UV LEDs, but they're pretty
expensive still.
Really the peak absorbance perfectly overlaps with the 254nm UV line from a low-pressure mercury lamp. As fancy, fun and fresh LEDs are, this really
looks like a lamp job to me
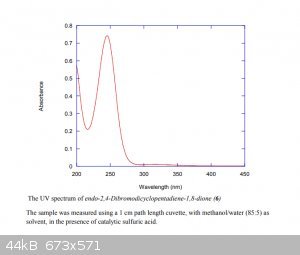
|
|
|
Quieraña
Harmless

Posts: 38
Registered: 24-8-2019
Member Is Offline
|
|
I saw this thing on yt about YAG crystal filled cubane molecules wrapped in tellurium or equivalent mirror shiny coating of nuclear unstable isotope
which, when beamed with xray became filled with infrared via YAG component which would , when injected say, to a cancer cell, be there knocking off
carcinomas via neutron bombardment AND a laser punch have ypur cake and eat it too, 'be rainbowed', channel on yt.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by Tsjerk  |
I was planning on doing that; but when I added the sulfuric acid to the toluene it turned black in minutes. I don't know if this happens to pure
toluene or that it is due to an impurity that is polymerizing. It does look a lot like tar and the color is captured by the sulfuric acid layer after
prolonged refluxing.
|
I think this is caused by the presence of methylthiophenes in petroleum-derived toluene, which cannot be separated by distillation. These compounds
are more readily sulfonated than toluene.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by DavidJR  |
I think this is caused by the presence of methylthiophenes in petroleum-derived toluene, which cannot be separated by distillation. These compounds
are more readily sulfonated than toluene. |
Indeed, I found out via this video. The method for purification shown in the video works nicely. I do think some p-TsOH is already formed at RT, as white fluffy crystals
crystallize when the toluene is added in a wet separatory funnel. Depending on the purpose of the toluene this can easily be removed with some
(bi)carbonate solution.
Quote: Originally posted by Tdep  | The wavelength has to be shorter than 350nm, which is frustrating, as most common UV LEDs are 365 nm. There's some deep UV LEDs, but they're pretty
expensive still.
Really the peak absorbance perfectly overlaps with the 254nm UV line from a low-pressure mercury lamp. As fancy, fun and fresh LEDs are, this really
looks like a lamp job to me |
I think I will order some of the cheap UV LEDs mentioned by Endo and hope they bleed enough into the <350 nm range. Maybe I can even ask the
supplier if he has an emission spectrum.
|
|
|
Housane
Hazard to Others
  
Posts: 127
Registered: 3-9-2018
Location: Worcester, England
Member Is Offline
Mood: Let’s make
|
|
Why do you want this Tsjerk? What idea do you have for it?
Green QD's so far
Feel free to correct grammar or incorect knknowledge. We are all learning.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
For the fun of it, it would be nice to have a NMR spectrum with that specific cubane hydrogen peak.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Philips makes some UV-B fluorescent tubes. Not cheap though. But maybe there's an affordable source near you, since Philips is a dutch company!
Otherwise, go with a mercury vapor lamp, high pressure if possible as it emits more UV-B.
I think almost any mercury vapor lamp is going to be better than any LED at those wavelength.
Mercury vapor lamp efficiencies are in the tens of percents, with multi-watts outputs, while deep UV-B and UV-C are less than a percent efficient, and
only emit milliwatts per LED.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Quote: Originally posted by Tsjerk  |
I think I will order some of the cheap UV LEDs mentioned by Endo and hope they bleed enough into the <350 nm range. Maybe I can even ask the
supplier if he has an emission spectrum. |
An emission spectrum is always nice to have, but I wouldn't expect them to bleed much at all into the 350nm range. The UV leds tend to peak rapidly
and then bleed into longer wavelengths a lot. So it probably has hardly any emission even at 360nm, but still a whole lot at 380nm. They tend to be
named after the shortest wavelength they produce in any meaningful way.
Also, as it heats up, it'll red shift some more, so if you plan to run them a long time, it'll get less efficient with time I believe.
Not meaning to rain on your parade on anything, I wish the absorbance spectrum at least showed some of the peak in at 360nm, then I'd be heaps more
keen for LEDs.
I've been looking at lamps on Amazon, and holy shit, what's with these prices? Is this some kind of chinese scam, or do they legitimately produce UV-C
radiation?? Crazy. Are they regulated? Link to product in picture: https://www.amazon.com/ultravioleta-germicida-esterilizador-...

|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Tsjerk: The emission spectrum is guaranteed to be in the datasheet of the LED. Have a look on digikey for some UV LEDs, for instance this one.
Tdep: These lamp really produce UV-C and in large quantities too! I bought a big 20W bulb for $15 a while ago and it produces a weird smell of burnt
skin when skin is exposed to it. It can excite calcium tungstate phosphor, which doesn't glow with UV-A. It's the real deal, and will damage your
eyesight without protection.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I found the perfect lamp! UV-B from Philips, peak at 310nm, 28 mm diameter so it would fit through a NS 29/32 neck. Only it costs around 40 euro...
These lamps are cheaper, but have their maximum intensity around 254nm. I could get one of these for around 10 euro.
What do you guys think? Would 254nm do the job? I don't know too much about light catalyzed reactions...
[Edited on 28-6-2020 by Tsjerk]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
254nm is perfect, that's what you want. You want the emission to overlap with the absorption of the compound, and that spectrum is what I posted
earlier in the thread, from the Supp info of the Pilot scale synthesis.
The absorbance peaks at ~245nm, so the emission of 254nm from the lamp is perfect.
Heptylene: That's amazing. I was wondering if they were some kind of fake product that's meant to capitalise on Corona fears... just strap a normal
lamp with some blue glass together or something... but it's amazing that they are real! I'm really keen to get one and try this out, this sounds like
a lot of fun. I'll want a big acrylic box or something for safety... hm
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Tdep  | 254nm is perfect, that's what you want. You want the emission to overlap with the absorption of the compound, and that spectrum is what I posted
earlier in the thread, from the Supp info of the Pilot scale synthesis.
The absorbance peaks at ~245nm, so the emission of 254nm from the lamp is perfect. |
Then I don't get this statement in the Falkiner article...
| Quote: | | The long reaction time for this process can be attributed principally to the polychromatic nature of medium-pressure Hg vapor lamps, with only minor
emissions in the critical region of 300 to 350 nm (see Supporting Information for UV spectrum of 6 under the conditions of the reaction). A better
matched light source would be expected to significantly reduce irradiation time, and the concomitant waste of energy. |
This is why I was looking for something between 300 and 350 nm.
|
|
|
| Pages:
1
2 |