| Pages:
1
2
3
4
5
6 |
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
It's not the speed of eU2A that interests me but the possibility of a constant pressure generator.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
It seems that a key goal here is achieving the ability to send the optimum air/ammonia mix to the catalytic reactor, smoothly and continuously.
With the air compressor you have control of the air feed rate. Now if you could match that with similar control over the ammonia feed you could
achieve that goal. The challenge is in how to do this.
You don't have a batch process here but a continuous process. This is much trickier to design and control. Industry would use flowmeters,
pressure controllers, and feedback loops. But for the home chemist the goal would be to do this in a much simpler and cheaper way.
Chemetix, you know all this. I'm just putting my thoughts on paper. You are doing a great job and I completely understand how time consuming all
this experimentation is. Like the rest of us you have other demands on your time. At least you live down under and don't have to face an ice-cold lab
like I do. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by Magpie  | | You are doing a great job and I completely understand how time consuming all this experimentation is. Like the rest of us you have other demands on
your time. |
Seconded. Your efforts are really appreciated!
Sometimes people ask me if I'm upset with them, because I seem argumentative. In actuality, they just did something that impressed me, so I'm trying
to draw out every detail so that it can be duplicated by others later on (hopefully without sounding too demanding). The challenge is to take the
piles of raw data (I added solution A to B, turned around and walked 3 feet in 0.25 seconds, waited 20 more seconds, sneezed, and then added some of B
to C), and isolate all of the important variables out of all that. Sometimes what's important isn't what you'd think.
I recently developed a procedure at work that did exactly what I wanted it to, every time. Then someone else tried to duplicate it, and got the exact
opposite results, every time. After some investigation, it was found that there was one step where I was rinsing a part with a spritz of DI water,
whereas the other person was rinsing it under running DI water for several minutes. It made all the difference in the results. So, we had to go back
and add a few ppm of "contamination" to get the desired results.
Another time, we developed a catalyst for a particular procedure. All of the samples worked more or less OK, but there was one sample that was
dropped onto the ground. It was carefully scooped up off the tile and put into a bag. Just for fun, this one was tested also. It turns out that
this one worked several times better than any of the others. Though a lot of effort was put into characterizing it, no one could find anything
different about that sample, other than its activity. So all of the experimental details were documented (drop sample onto floor, dance in a circle,
scoop up this way with this spatula, etc.). Maybe some day someone else will be able to take this information and make sense of the results.
A general rule around my lab is, the most useful bit of documentation is the part that didn't get recorded. Additionally, experimental details that
I could expertly recall during an experiment, are a week later just a vague memory. Finally, too much caffeine brings on hallucinations of pet
unicorns and talking cats.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
The attention to details is what makes chemistry an art. Physicists take their superiority from working with first principles and mathematical logic.
Chemistry gets its results from the messy chaotic world of reality. When you are trying to replicate a published work and you stand there after the
third hour of slow addition with stirring, and you contemplate that the author reports, ' ...after half an hour the mixture becomes milky and changes
viscosity...' while you are looking at a clear reaction.
Are you able to know that what you are looking at has all the signs of the reaction still working fine despite the obvious difference from what was
reported. Are you able to draw on your dark arts of the work up and get the product and yield despite doing it differently from the published work.
Are you able to intuit that because you used a technical grade reagent the impurity prevents the phases from separating like it was published.
And this is why I love running my own research projects, no lab manager or supervisor insisting I do it a certain way when I know I can cut out three
steps. B.P-be damned!
And I love SM, being able to publish my work in almost real time. To peers and what seems like lab partners you can lean across the bench and say '
Hey, check this out' to.
Without the lab politics, time pressures, constantly justifying everything you do, staying on an agreed path of work when you know a detour might
solve an impasse.
There are some real benefits to being a backyarder. And some real detriments. Being poor and not having the LC-MS you fantasize about putting in the
corner where you just know it would fit. or a lab that is either freezing or sweltering. (I've had a few days recently where it just got too hot to
work)
I'll continue to fill in as many details as possible. But I should have been clear about my mission statement much sooner. The project was focused on
' Make nitric acid from urea in a way that can be easily replicated '.
So in many ways I'm being deliberately rough, no measured amounts no specific grades of materials. And can I, under these conditions, have a robust
plant that can still produce useful amounts to those who need only 100ml.
I might offend the research chemists, but I will have more appeal to those who are looking for a way of reliably obtaining their much coveted reagent.
If I can achieve that, then I'll think about doing some kinetic studies on the alternative catalysts and publish their relative effectiveness. Or,
someone else can, I'm not protective about this work.
In fact working collectively and openly justifies our existence as amateurs. We can generate good chemistry from a community model not a capitalist
one.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
FYI, here's a schematic of a fluid control system I have found useful. It shows a way to control the feed rate using a valve located on a bleed line.
This example is for control of an air feed. But I use it most of the time to control the amount of water sent to my condenser.
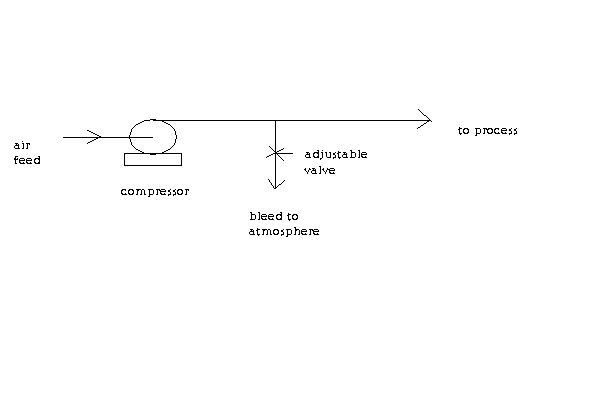
[Edited on 29-12-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Very, very interesting, thanks! For some, this extremely useful reagent is very difficult to obtain. Seems worth a sticky to me.
| Quote: | | So in many ways I'm being deliberately rough, no measured amounts no specific grades of materials. And can I, under these conditions, have a robust
plant that can still produce useful amounts to those who need only 100ml. |
There is definitely something to say for this philosophy also in a professional setting. I have a colleague that changes seemingly unimportant
variables in experiments all the time when 'duplicating' it a second or third time. He deliberately takes the other bottle of a reagent if we have
several of them in the storage room, uses a different pipette, takes a coffee break in the middle of the experiment, etc. His reasoning is that if an
experiment is robust to these kinds of changes, it is more likely that it can be duplicated by someone else in a different lab. Most scientists would
try to replicate the conditions of their initial experiment as closely as possible and only if time permits proceed to try different variations. Time
being a very valuable resource however, this step is skipped more often than not.
I tried this reaction also using copper wire, but the heat of the reaction is sufficient to melt the copper wire very quickly, even without applying
external heating. Do you adjust the heating to compensate for the heat generated by the exothermic reaction itself?
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Is there a risk of explosion inside the glassware?
NH3 + O2 + catalyst may be used as rocket propellant.
[Edited on 30-12-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I like your philosophy. For amateur chemistry supplies and equipment are often a limiting factor, one mans "simple build" can be an acquisition
nightmare for others.
This process seems quite robust, and the different parts can easily be modified to suit individual preferences and capabilities.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Schematic diagram
I guess if there was pure ammonia and pure oxygen it's a potential risk. I'm fairly sure that ammonia in air and diluted with CO2 is highly unlikely
to ever ignite back upstream.
I think I said earlier that I'd draw up a schematic.
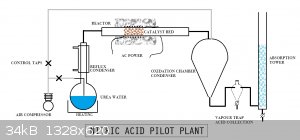
[Edited on 30-12-2016 by Chemetix]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice! Thanks for this. I'm loving the simplicity.
Wiki says that the explosive limits in air are 15-28% NH3. The somewhat heated (I presume) air/NH3 mix coming out of the NH3 generator is diluted with
CO2 and water vapor. Are there any scenarios where explosion is a risk that you are aware of at this time?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
You know, I tried to see how you could blow up the system and I don't think it's easy. If you managed to fill the biggest volume with air ammonia, and
somehow managed to ignite it, it burns so slowly without a catalyst, so the worst that could happen is you pop the top on the sep funnel. I saw a wisp
of blueish flame try to creep back from the copper oxidised reaction once, but it stopped suddenly. I read somewhere that ammonia is difficult to
sustain combustion even under perfect conditions.
Wiki confirms this:
"The combustion of ammonia in air is very difficult in the absence of a catalyst (such as platinum gauze or warm chromium(III) oxide), because the
temperature of the flame is usually lower than the ignition temperature of the ammonia–air mixture. The flammable range of ammonia in air is
16–25%.[22]"
As far as a typical fuel air mixture goes, this one seems fairly tame.
And by the way- Ostwald got his process, Haber got his process, then there's Solvay, Weldon, Burton, Raschig, Andrussow....to name a few. Does this
constitute a unique process by industrial standards? Do I get to name it?
[Edited on 31-12-2016 by Chemetix]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
In the past I have done my experimentation on the ostwald process, and got success. I was, however, using platinum. As a substrate I would recommend
either asbestos or the sintered oxide itself.
To generate a continuous flow of ammonia, the best would be to prepare it and store it under pressure with a flow regulating valve, however, since
this is inconvenient (in my personal experience at least) Direct production is more desirable.
One could make a setup in which urea is decomposed, the gas passed through triethanolamine and the resulting gas should be quite pure ammonia. A flow
meter could then be installed to monitor the output.
A side note about the air/ammonia ratio: In my experimentation and research I found out that if too much ammonia is present one would only get
nitrogen and water because ammonium nitrite would be formed _in situe_. On the other side, if too much oxygen is present, the nitrogen oxides tend to
decompose back into oxygen and nitrogen. One should try to maintain a slight oxygen excess for better yield of the nitrogen oxides and put another air
inlet after the catalyst tube.
For optimal absorption in an amateur setting, 3 column in a row can be used. A strong cooling of the exit gases and the tower is recommended for
superior efficiency.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
" One should try to maintain a slight oxygen excess for better yield of the nitrogen oxides and put another air inlet after the catalyst tube.
For optimal absorption in an amateur setting, 3 column in a row can be used. A strong cooling of the exit gases and the tower is recommended for
superior efficiency. "
That's good advice.
Would you recommend having the highest concentration of catalyst per surface area and generating the highest reaction temperatures you can, or as I
have been doing, diluting the catalyst over a larger area and running at a lower temperature than would be seen in conventional Ostwald plants?
I will aim to have some form of monitoring system of the gas composition before and after the reaction zone. I hope it's not necessary for a good
yield. That there will be a fairly wide sweet spot for optimum production. One that is easy to stay within for an amateur and still get, say, 80%
yield based on the urea. I suspect it's possible but like most things, they turn out differently in practise.
[Edited on 31-12-2016 by Chemetix]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Well, try to limit the flow to keep the temperature on point with a concentrated catalyst.
As for the yield, I'm afraid it won't exactly be what you expect. Thermodynamics alone makes it hard to reach your expected yield. By the design of
the process alone you will lose a good deal of ammonia as hydrogen/nitrogen/water. In the disproportion of the nitrogen oxides you will also loose a
fair bit.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
While it's good to cover all the points I don't think a gas explosion is very likely. Ammonia isn't very flammable and small diameter tubes reduce the
risk further. A flame arrester made from metal mesh could be installed if you're worried, but I don't think it's necessary with this
ammonia/CO2-mixture.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
@chemetix
I am assuming that you have already come across the (rather lengthy) thread -- the Drunken aga challenge for producing nitric acid. If not, here it
is: https://www.sciencemadness.org/whisper/viewthread.php?tid=48...
One of the most promising ideas to come from that was a gem of a paper that deltaH found. His link seems to be broken but I found an alternative: https://www.forgottenbooks.com/en/books/ManganeseintheCataly...
[edit: only part of the paper available. Jump forward two posts for actual file. /edit]
As you can tell from the title, manganese dioxide has yielded results in this process before. I just thought I would throw this idea in the hat for
when we get to playing with different catalysts. MnO2 has to be one of the most accessible chemicals around and if it can be made to work reliably I
think it would meet your overall aim.
I think that your apparatus is vastly simpler than the setup that Snowden Piggot came up with.
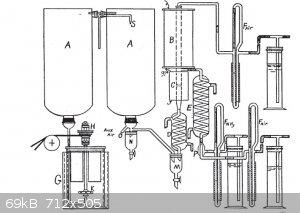
[Edited on 1-1-2017 by j_sum1]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
The apparatus will be as complex as funds and supply allows 
(Nerd rules applies).
[Edited on 1-1-17 by Fulmen]
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
" MnO2 has to be one of the most accessible chemicals around and if it can be made to work reliably I think it would meet your overall aim."
I suspected it might work, and I might just have to open an old dry cell just to see.
Jsum, that apparatus from Piggot seems like a liquid reaction scheme, i'd be very interested in seeing the details. The link to the book gave an
excerpt that said something about preventing inhalation from carbon monoxide...I'm confused.
Today I had the inevitable run that seemed to not work as well as the last few. I changed the level of doping on the substrate this time to make a
higher concentration and it didn't seem to react as hot or as fast as the last time I tried nickel oxide.
Will try again soon.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Strange.
Ok... here is the actual paper.
Attachment: manganeseincatal00piggrich.pdf (1.3MB)
This file has been downloaded 1307 times
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Try to use nickel nitrate impregnated catalyst and reduce it in the tube with the usual procedure, the catalyst is going to be much more active this
way. Also, ammonia/air explosion can happen, however it's extremely unlikely, plus, it doesn't combust very fast. If you are worried, put a pressure
check blow valve if you increase the pressure. It could literally save your life.
[Edited on 2-1-2017 by plante1999]
I never asked for this.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Thanks Jsum. The Piggot paper is a very similar scheme to what I have just built. I'm glad to have this, it pretty much outlines the problems faced
with the oxide catalysts. Variable activity and quite sensitive to how they are run. After reading this it struck me that changing how you start the
reactor could affect the effectiveness of the catalyst. And a detail that might explain why this run was quite different from last time. I waited for
the ammonia generator to come up to temperature and production before introducing it with air to the fresh catalyst. The last few runs the catalyst
was heated, then the air was started and at the same time the ammonia generator switched on. The rate of ammonia slowly increasing up to about 6% the
total volume. The slow introduction of ammonia could be critical to how the oxide performs as a catalyst.
@Plante " use nickel nitrate impregnated catalyst and reduce it in the tube "
I'm curious, if platinum and copper work as a catalyst while metallic, do the oxides work as true oxides or do they get reduced to a metallic state?
2NH3 + 3MO => 3M + N2 + 3H2O
I might run some hydrogen through the tube at heat to reduce the oxide, then see how it behaves.
First I'll replicate the slow startup procedure and see if this is a variable.
See below; I like how I'm able to edit the post in the past.
[Edited on 1-1-2017 by Chemetix]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Fascinating read. It's becoming apparent that many metal oxides can work as a catalyst, but most plants still use platinum. So it's safe to assume you
are working with a disadvantage. If readily available oxides were great we'd seen more commercial use by now. But for amateur use it still seems
promising, as long as the catalyst isn't expensive or hard to make a bit of wear might not be a major issue. Nobody's going to run such plants for
days anyway.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Well, in the case of copper, it's the oxide/metal doing the work. Just like with silver, or many other metal catalysts.
My knowledge of what goes on exactly with platinum is too limited to tell you what happens then.
However most catalyst of this nature work by a constant reduction/oxidation. You can see this in action by putting copper wire over an acetone beaker.
If you heat the wire and lower it you will notice it starts glowing from the heat and move from "black oxide" to "copper" back and forth.
Calcination is better done under an oxidative atmosphere for these catalyst. If you put ammonia in you could get nitrides, although this is far
fetched. Mainly the porosity will be lowered.
The thing that you want is active oxides. In the case of copper, it's easy to get activity is not very surprising considering the purity in which you
can get copper, and the metal properties.
I never asked for this.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
The mechanism of catalysis is of course a complicated affair, platinum seems to work by pulling off hydrogen from the moiety where copper seems to
offer an active oxygen. It's the idea that the metal does the transfer of electrons while the oxide becomes electron deficient and activated that is
the way I see this working. Whether this is correct is speculation on my behalf at this stage. But I see this is where the activity of the oxide
catalyst comes from, small sections of the oxide have exposed metallic sites to transfer electrons.
I tried the same start procedure as earlier than yesterdays run and it seems like there are subtle differences. The catalyst seems to build to a
maximum efficiency where as the quick start seems to have a lower stable level of efficiency. I really need to have some analytical hardware to make
these calls, but for now perception is all I can go on.
But todays' run with a new catalyst made with 10g light tan house brick support and 1.0g NiO filling the tube to 5cm, ran pretty much the same as
yesterdays run. This time it ran continuous for 5 hrs and generated 13ml of condensate and 15ml of tower absorption. I realised I have no indicator on
hand, otherwise they would have been titrated. An estimate based on the way they took a certain amount of bicarb to neutralise would be, condensate
50% conc. - tower solutions 30-40%.
The urea is so slow to decompose, I think doing your gas in a bag trick WGTR is the way to get the controlled flow and more of it. I am torn with the
electro-accelerated decomposition scheme, sounds effective, but do I want more complicated equipment to build...not at the moment. Maybe I'll just get
a 10 litre pot and fill most of it with urea and water and bang on a reflux system, stick the whole thing on a gas stove.
I wonder if copper could be 'poisoned' to prevent it over oxidising the ammonia back to N2.? Lindlars version of copper.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
That's understandable, you have more than enough to deal with at the moment. Still, for a plant capable of producing practical amounts of acid a
better method for ammonia is needed. Perhaps someone else could start experimenting with the eU2A, by the time they have a working method I'm sure
you've made headway with the catalyst.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
| Pages:
1
2
3
4
5
6 |