| Pages:
1
2 |
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
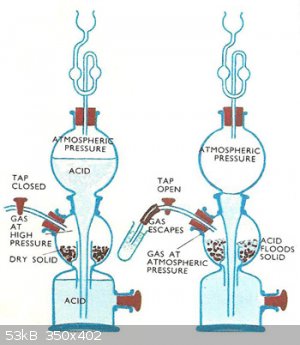
I think someone already solved this problem in 1844.
https://en.wikipedia.org/wiki/Kipp%27s_apparatus
[Edited on 29-11-2017 by VSEPR_VOID]
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I bet it was expensive to acquire one in 1844 too.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Kipp's apparatus is very clever, however it doesn't guarantee a constant gas flow rate, which is kinda needed in a GC column.
Another problem with it is Leaks or Blockages.
A blockage is kinda fine, but a leak would prevent it from working to stop the gas production.
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Quote: Originally posted by aga  | What reaction conditions did you use ?
Al powder, chips, solid chunks ?
What NaOH did you use ? Solution or dry NaOH on the metal with water dripped on ?
Science doesn't happen by vagueness.
Details and data or it's like a fart in the breeze,
. |
1: I wasn't doing science, I was making floaty balloons for my kids
2: NaOH solution (somewhere around 100g to a litre of water - it wasn't for science), Al chunks - usually long pieces of scrap extrusion.
3: Surely any observations about the reaction are useful in designing a method to overcome potential problems?
Personally I think you have been too quick to abandon electrolysis, given the ease of control, and low cost of consumables.
| Quote: |
Hydrogen will also be needed in much higher quantities to produce the flame for the FID
|
and this is where electrolysis wins. Its used to produce H2 fast enough for welding torches.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
If you do the maths, the energy required to make enough hydrogen for those torches is enormous.
Water really likes to be water, so it takes a LOT of energy to break those H-O bonds.
Others have also said it would be cheaper/easier to buy a second-hand GC, and it would.
What would be the point though ?
Personally i would like to know how one works from the ground up and make one.
|
|
|
GrayGhost-
Hazard to Self
 
Posts: 61
Registered: 31-10-2017
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
I build a homemade Kipp apparatus for chlorine. here link of model
https://makezine.com/projects/on-demand-benchtop-gas-generat...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nice design !
|
|
|
Leafs
Harmless

Posts: 19
Registered: 30-7-2017
Member Is Offline
Mood: No Mood
|
|
I produced hydrogen gas by slowly dripping NaOH solution on aluminum foil balls in water and the vessel in an ice bath. The gas produced was passed
through a drying tube with calcium chloride. You could fill up balloons but if you need to hydrogenate something I found it more effective to fill a
jimmy rigged beach ball (that was completely evacuated with a vacuum aspirator) and use that to fill balloons. Did not collect the initial gas
produced and waited until it lit a flame consistently to collect.
Indeed you gotta make sure you keep temperature under control or things can runaway very fast, which actually happened the first time lol. Also I
think it's best to go with an excess of aluminum because when I went with molar equivalents I ended up having leftover NaOH, so the foil may of been
impure or some other factor.
I noticed the hydrogen produced had this faint smokeyness to it. Not exactly sure what that byproduct was because it should've been colorless and
undetectable. If anyone has a guess let me know because the following hydrogenation did work.
Have also attempted to produce via electrolysis but ended up with severe corrosion of the copper cable leading to the carbon electrodes, and very slow
rate of hydrogen production.
Calcium hydride apparently isn't that easy to source (cheaply) these days for the amateur. Even the chinese want an arm and a leg, mostly on
shipping.
[Edited on 30-11-2017 by Leafs]
|
|
|
| Pages:
1
2 |