HCl, would come from both reactants.
HCl04, thermodynamically, would not favour such a reaction (at normal temps.). (Guanidine HCl04, is highly water soluble, thus no exothermic heat of ppt. to drive the reaction, either) .
Thus, displacement of HCl by HClO4, is most unlikely.
Along with HCl, you volatised gas, surely a mix of N2, NxOy, H20, CxOy.
However, heating HCl04 with Guanidine HCl, pushes Gibb's Free energy, in a dangerous direction.
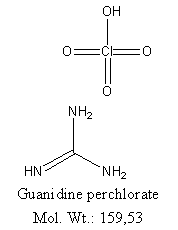
Perhaps, since you are a moderator, the Guanidine HCl04 discussion, is best left with the geeks at NASA.
My thanks for the interaction.