Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Solubility of guanidine perchlorate?
Has someone any idea about the solubility of guanidine perchlorate [CAS 10308-84-6]. This chemical is very seldom, so that’s the reason why I
can’t find any solubility-data. I checked Ullmann’s Encyclopedia of Industrial Chemistry, the Merck Index and Römpp (a very good german
chemistry-lexicon), but without success. If nobody can’t find any solubility-data, so maybe someone can make predictions about its solubility.
Here the structure (made with ChemDraw):
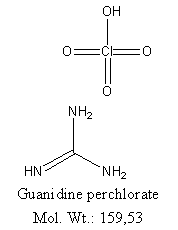
|
|
|
TitusGabonicus
Harmless

Posts: 11
Registered: 11-5-2011
Location: UK
Member Is Offline
Mood: No Mood
|
|
Guanidine Perchlorate
No.
However, I recently found an apparent double-decomposition synthesis, in 'Explosives'. {apologies, I will add author tomorrow (can't sleep for
counting formulas night)}?
I found it a particularly exacting text, though it stated only... G.P. can be synthesised by reacting sodium perchlorate and guanidine hydrochloride.
Whether one gets a ppt. of NaCl (since both reactants are highly water soluble, with a conc. solution of Guanidine.HClO4, I am unsure, or conversely,
it is quite insoluble?).
Thus, to date I have chosen not to test. I remember the lead-block test to be 420cm-3 (!0g sample).
It further stated it to be highly shock sensitive, & extremely friction sensitive.
I shall scan details, rather than quote, at this hour of the morn.
Theoretically, it looks pretty awesome, too.
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This compound is very soluble in water. I tried this myself by mixing a concentrated solution of guanidinium chloride with 35% perchloric acid. No
precipitate is formed at all. On boiling down and driving off hydrogen chloride still no precipitate is formed.
|
|
|
TitusGabonicus
Harmless

Posts: 11
Registered: 11-5-2011
Location: UK
Member Is Offline
Mood: No Mood
|
|
The text above with GP Prep. Was:-, 'Explosives' - Author: Rudolph Meyer et.al, 6th ed. Pub. 2007. ISBN-978-3-527-31656-4. (Printed on acid-free
paper, for those it may interest!)
It is now back in the Uni library. ‘Google books’ edition, stopped at Ethylphenylurethane, & the book is arranged encyclopaedically.
Shock & friction sensitivities, were roughly 4N & 0.1N, respectively.
I had a little ‘Oogle’ today, and thank you for the response.
I can’t think 'why', but I am not surprised that particular method failed. Perhaps someone may enlighten further.
I do however, tend towards the view, GP, is a highly soluble salt.
Personally, it can remain that way, since with its extreme sensitivity & ferocity, best left to greater/and/or more stupid men than I.
However, these compounds (i.e ‘nitramines‘), are possibly the future in rocket chemistry???.
|
|
|
TitusGabonicus
Harmless

Posts: 11
Registered: 11-5-2011
Location: UK
Member Is Offline
Mood: No Mood
|
|
Guanidine HCl + HCl04 -> ????
HCl, would come from both reactants.
HCl04, thermodynamically, would not favour such a reaction (at normal temps.). (Guanidine HCl04, is highly water soluble, thus no exothermic heat of
ppt. to drive the reaction, either) .
Thus, displacement of HCl by HClO4, is most unlikely.
Along with HCl, you volatised gas, surely a mix of N2, NxOy, H20, CxOy.
However, heating HCl04 with Guanidine HCl, pushes Gibb's Free energy, in a dangerous direction.
Perhaps, since you are a moderator, the Guanidine HCl04 discussion, is best left with the geeks at NASA.
My thanks for the interaction.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Guanidine Hydrochloride is soluble in hot, nearly boiling methanol at about 50% by weight of the solution.
Sodium Perchlorate is methanol soluble at 25C to about 33% by weight of solution. Nearly identical solubility at 25C for acetone.
Ammonium Perchlorate is methanol soluble at 25C to about 6.4% by weight of solution. About one third that solubility at 25C in acetone.
Sodium Chloride solubility at 25C in methanol is low at about 1.4% and almost insoluble in acetone.
What is the solubility of Guanidine Perchlorate is the solvents is unknown.
However it appears methanol and acetone could be useful in formulating a scheme to separate the byproduct sodium chloride.
|
|
|
TitusGabonicus
Harmless

Posts: 11
Registered: 11-5-2011
Location: UK
Member Is Offline
Mood: No Mood
|
|
Guanidine nitrate actually brought me to this topic.
Jetex motors, & their fuels, used for model planes etc. have recently become 'vogue'. (See www.jetex.org.com)
The fuel 'pellets', were principally manufactured by ICI.
Initial reading referred to GN + nitrated resorcinol’s, as a 90:10 % w/w mix.
My initial hunch + calcs, pointed surprisingly, but correctly, to 2, 4,- Dinitrorescorcinol, as the principle fuel.
ICI Patents, on the Jetex site, relating to fuel research, reveals a variety of mixtures based around this formula.
Many other dinitro compounds, with pesticide applications or other agricultural toxins, were tested. (Explosives & Agro-Chem., then ICI's bread
& butter.)
Having dealt with ICI, & Agro Toxins in the past, plus studies in bio-toxin application, it made more sense the toy planes were a research profit
raising offshoot. The use of 'Jetex' type motors, in larger radio-controlled pesticide applicators, was 'vogue' at that time
(One mix was GN 60%, 2,4,-DNR 15% + DDT 15%).
The combustion temp. is stated to be little more than 300 Degrees Celsius (?), thus decomposition of these useful AgroChem's, was potentially minimal.
Also, it was both safer, & cheaper, than using large planes w/ Pilots.
Another driving force was the military application of such devices.
(Hydrazine, too, was also widely researched in the late 80's by USAF).
Thus, those little Jetex 50 ‘toy’ motors, and fuels, now 'Health & Safety' 'rapid watery-eye movements', were probably ‘fuelled’ (sic.),
by both the Agro-Chem. Industry & The Military.
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by TitusGabonicus  | Guanidine HCl + HCl04 -> ????
HCl, would come from both reactants.
HCl04, thermodynamically, would not favour such a reaction (at normal temps.). (Guanidine HCl04, is highly water soluble, thus no exothermic heat of
ppt. to drive the reaction, either) .
Thus, displacement of HCl by HClO4, is most unlikely.
Along with HCl, you volatised gas, surely a mix of N2, NxOy, H20, CxOy.
However, heating HCl04 with Guanidine HCl, pushes Gibb's Free energy, in a dangerous direction.
Perhaps, since you are a moderator, the Guanidine HCl04 discussion, is best left with the geeks at NASA.
My thanks for the interaction.
|
From your response I get the idea that you do not understand what guanidinium chloride really is. There is
NO free HCl in th solution of this compound. Guanidine is a rather strong base and guanidine HCl almost completely consists of guanidinium ions
[C(NH2)3](+) and chloride ions. With HClO4 you bring in H(+) and ClO4(-) ions and if guanidinium perchlorate were only marginally soluble, then
certainly you would get a precipitate of the perchlorate. I already used this method of preparation for other other organic perchlorate salts, by
simply adding a dilute solution of HClO4 to th solution of the organic chloride. E.g. trimethylammonium chloride with dilute HClO4 gives a precipitate
of trimethylammonium perchlorate. The same works for the tetramethylammonium salt. In solution, HCl remains behind. So, with guanidinium chloride this
method of reasoning is not strange at all and I think that on boiling down and driving off all HCl you can obtain the perchlorate salt, but I did not
try that, because I do not want to risk an explosion or very violent decomposition of a hot organic perchlorate salt.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not know the solubility for guanidine perchlorate in water, but guanidine sulfate is almost insoluble in pure alcohol.
There is another interesting exception to the general solubility rules. While ammonium salts and salts of organic amines usually tend to be very
soluble in water, the perchlorates of organic amines tend to have much lower solubilities. Indeed, ammonium perchlorate is much less soluble than
sodium perchlorate (usually it is ammonium salts that are more soluble than sodium salts), although potassium perchlorate is less soluble than either.
So one would think that guanidine perchlorate would only be moderately soluble in water. On the other hand, guanidine salts do have two other amine
groups, and this would tend to increase the solubility.
[Edited on 7-2-2012 by AndersHoveland]
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by AndersHoveland  | | [...]Indeed, ammonium perchlorate is much less soluble than potassium perchlorate (usually it is ammonium salts that are more soluble than potassium
salts). |
This is absolutely not true. Ammonium perchlorate is MUCH more soluble than potassium perchlorate.
Potassium perchlorate is only very sparingly soluble in cold water (just a few tenths of gram per 100 ml of water), while ammonium perchlorate
dissolves quite well (if I remember correctly, well over 10 grams per 100 ml).
The very low solubility of KClO4 in cold water, combined with its decent solubility in boiling water makes isolation and purification of KClO4 very
easy. Just boil the water, add as much of (impure) KClO4 as possible such that all of it just dissolves and then allow to cool slowly: nearly 100% of
the KClO4 crystallizes and impurities remain in solution.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by AndersHoveland  | I do not know the solubility for guanidine perchlorate in water, but guanidine sulfate is almost insoluble in pure alcohol.
There is another interesting exception to the general solubility rules. While ammonium salts and salts of organic amines usually tend to be very
soluble in water, the perchlorates of organic amines tend to have much lower solubilities. Indeed, ammonium perchlorate is much less soluble than
potassium perchlorate (usually it is ammonium salts that are more soluble than potassium salts).
So one would think that guanidine perchlorate would only be moderately soluble in water. |
Actually at 25C in H2O, Ammonium Perchlorate is ten times as soluble (20%) as Potassium Perchlorate (2%)
Hehehe ......that's why woelen's avatar is "Adminstrator" .......
and regarding the avatar for AndersHoveland .....
well what more can really be said 
Okay I'll take a shot at it, even at the risk of it being
regarded as a cheap shot ....it truly isn't meant that way.
How about this ....Anders "seems to speak"
authoritatively and knowledgeably when actually not truly knowing
what he is talking about with disturbingly perennial regularity .....
(not that anyone is 100% correct all of the time)
and it isn't a problem that Anders doesn't know so much but rather
the problem is that so much of what Anders knows is wrong.....
but this never seems to slow down the flow of the river of shit.
[Edited on 7-2-2012 by Rosco Bodine]
Attachment: Perchlorates SOLUBILITY .pdf (727kB)
This file has been downloaded 1186 times
|
|
|
TitusGabonicus
Harmless

Posts: 11
Registered: 11-5-2011
Location: UK
Member Is Offline
Mood: No Mood
|
|
Thank you kindly for the pdf. of ClO4- solubilities. it is proving to be a very interesting read.
I don't want to draw this topic out unnecessaily, nor do I confess be an expert on Guanidines. Far from it. They have become a curiosty to me in my
old-age, moving into onco-pharm. rather than hydrazine research, in my youth. Something I possibly regret!?.
One thing is clear, were there a solubilty coefficient, which could be applied to both solvent/solute alike, Chemistry would, well, ???????.
However, the trends mentioned above, do make good 'rules of thumb'.
I am aware Guanidine forms salts, akin to the ammonium ion, & differing from urea.
Thermodyamics too, is my 'anorak' subject, its true,(i.e passes time, writing down meaningless numbers, I can study later!)
I certainly do not understand Guanidines. I missed them first time around, when they were of less interest. I am now enjoying my attempts to
understand them, & possible future trends?
I have really enjoyed interacting with the people this post, my thanks,
However, if I may throw one last fly in the ointment (Spanish Cantharides!), couls the chlorate be made boiling with Ghcl, or ppt. the MnO4- with?. I
feel sad it can't form a peroxide, as does urea?
|
|
|
TitusGabonicus
Harmless

Posts: 11
Registered: 11-5-2011
Location: UK
Member Is Offline
Mood: No Mood
|
|
My apologies.
 
Courtesy of

|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Most of the gold which exists on earth is dissolved in seawater.
|
|
|
siegfried
Harmless

Posts: 18
Registered: 15-8-2012
Member Is Offline
Mood: No Mood
|
|
If you can't get guanidine carbonate to react with perchloric acid because you can't buy either one, maybe guanidine chloride and sodium perchlorate
would work. Guanidine perchlorate would precipitate out of the chloride/perchlorate solution after a time in the freezer and you would be left with a
solution of NaCl and solid guanidine perchlorate. I have seen the chloride on Ebay from some Korean Chemical Co, but it is rather expensive.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|

@ siegfried
Thank Rosco Bodine for this _
http://www.sciencemadness.org/talk/viewthread.php?tid=8911#p...
Preparation of Guanidine Salts (Guanidine Chloride) US278327
Attachment: Guanidine Chloride US2783276.pdf (212kB)
This file has been downloaded 689 times
To make (H2N)2CNH • HClO4 , mix together with the stoichiometric amount of NaClO4 or KClO4
(H2N)2CNH • HCl + NaClO4 => NaCl + (NH2)2CO • HClO4

|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Sorry , that last line above here is not Urea of course but rather , Guanidine
(H2N)2CNH • HCl + NaClO4 => NaCl + (H2N)2CNH • HClO4
.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Hi,
I just got some guanidine hydrochloride, I wonder if i can make the perchlorate of guanidine from sodium lithium or ammonium perchlorate simply by ion
exchanging ?
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Isolating the perchlorate is not easy, guanidinium perchlorate is very soluble and very hygroscopic and hence not easily isolated. Allowing a solution
of this compound to dry at room temperature does not work, it simply does not form crystals, it remains a liquid, due to adhering water.
|
|
|