
GadZooks - 5-2-2018 at 18:58
I recently got some tritium vials to go into a wedding ring. I hope to be wearing this thing for a few decades, so I'll almost certainly see the vial
reach the end of its useful life. I imagine I'll replace the vial or the whole ring, but I had a thought after reading an interesting fact: Tritium's
decay product, 3He, can be converted back into tritium through neutron capture.
I happen to be going to a university that has an Am-Be thermal neutron source. I've done some neutron activation analysis for an undergraduate lab. My
question is, could I (safely) recharge my ring by placing it in the neutron flux?
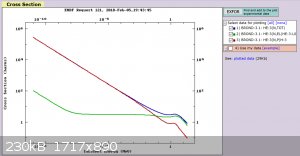
I've done a little bit of research so far, but I haven't had time yet to sit down and do a calculation. The cross section for the 3He (n,p)
T reaction for thermal neutrons (<0.025 eV) is 104 - 105 barns (see picture). For a 1.5x5mm vial, the activity at sealing is
21 mCi. Let's say I let it decay for one half-life before putting it into the thermal neutron source. What flux would be needed to get it back to ~70%
of its original tritium content?
I haven't yet looked at what the tritium, glass, phosphor, or ring would do with the neutrons. The ring would be a silver alloy, which would be hot
for at least a few weeks after. I would love to know what the phosphors used in the vials are made of so I can take into account any activation
products there.
I'm mostly putting this here as a placeholder for my thoughts so I can come back to it, but I'd be interested in anyone else's thoughts!
3He cross section: www-nds.iaea.org
Tritium activity: http://www.candlepowerforums.com/vb/showthread.php?399475-B-...
phlogiston - 6-2-2018 at 06:15
If metastable Ag-108m forms, then it will be hot a lot longer (halflife 418 y)
Also, silicon-32 (the glass probably contains Si) could be a long-lasting problem (153 years).
IIRC, copper and phosphorus don't have unstable isotopes that will last years.
Interesting idea, but I doubt you will be able to generate a useful level of tritium in this way. Not going to calculate it though.
MrHomeScientist - 6-2-2018 at 06:27
Look at Hal Jordan over here, trying to recharge his glowing green ring 
Really interesting idea, but I agree that radiation of the entire ring could cause unforeseen problems. Can you take the vials out and charge them
separately? I'd love to see a picture of the ring, too; it sounds awesome.
SWIM - 6-2-2018 at 21:23
That reaction releases what... 0.764 Mev?
Won't it get a little warm?
That looks like a fair bit of energy per nucleon to me.
Bert - 6-2-2018 at 22:12
https://m.banggood.com/1_5x6mm-Trit-Vials-for-DQG-SPY-DQG-Fa...
https://m.banggood.com/1PCS-2x12mm-Multicolor-Tritium-Vials-...
https://m.banggood.com/3x22_5mm-Trit-Vials-Tritium-Self-lumi...
https://m.banggood.com/1pcs-Trit-Vials-Tritium-Self-luminous...
These might be a little cheaper, faster, safer? But not nearly as likely to give you super powers!
wg48 - 7-2-2018 at 04:23
They used to be put in the dials (a round thing with ten finger holes used for dialing LOL) of trim phones (first modern British phone). So you could
dial in the dark. They had special evacuation procedures in the event that a box of vials being damaged

Thats way back near the end of the electromachanical days of telephony mid 1960s. When some director (a machine to decode the dialled area code in to
a routing code) centres had hundreds of mostly young female telephonists sat at long rows of switch boards and it was the sixties.
[Edited on 7-2-2018 by wg48]

