GadZooks
Harmless

Posts: 1
Registered: 5-2-2018
Member Is Offline
Mood: No Mood
|
|
Tritium vial "recharge" by neutron activation (speculative)
I recently got some tritium vials to go into a wedding ring. I hope to be wearing this thing for a few decades, so I'll almost certainly see the vial
reach the end of its useful life. I imagine I'll replace the vial or the whole ring, but I had a thought after reading an interesting fact: Tritium's
decay product, 3He, can be converted back into tritium through neutron capture.
I happen to be going to a university that has an Am-Be thermal neutron source. I've done some neutron activation analysis for an undergraduate lab. My
question is, could I (safely) recharge my ring by placing it in the neutron flux?
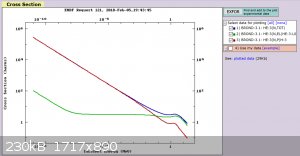
I've done a little bit of research so far, but I haven't had time yet to sit down and do a calculation. The cross section for the 3He (n,p)
T reaction for thermal neutrons (<0.025 eV) is 104 - 105 barns (see picture). For a 1.5x5mm vial, the activity at sealing is
21 mCi. Let's say I let it decay for one half-life before putting it into the thermal neutron source. What flux would be needed to get it back to ~70%
of its original tritium content?
I haven't yet looked at what the tritium, glass, phosphor, or ring would do with the neutrons. The ring would be a silver alloy, which would be hot
for at least a few weeks after. I would love to know what the phosphors used in the vials are made of so I can take into account any activation
products there.
I'm mostly putting this here as a placeholder for my thoughts so I can come back to it, but I'd be interested in anyone else's thoughts!
3He cross section: www-nds.iaea.org
Tritium activity: http://www.candlepowerforums.com/vb/showthread.php?399475-B-...
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
If metastable Ag-108m forms, then it will be hot a lot longer (halflife 418 y)
Also, silicon-32 (the glass probably contains Si) could be a long-lasting problem (153 years).
IIRC, copper and phosphorus don't have unstable isotopes that will last years.
Interesting idea, but I doubt you will be able to generate a useful level of tritium in this way. Not going to calculate it though.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Look at Hal Jordan over here, trying to recharge his glowing green ring 
Really interesting idea, but I agree that radiation of the entire ring could cause unforeseen problems. Can you take the vials out and charge them
separately? I'd love to see a picture of the ring, too; it sounds awesome.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
That reaction releases what... 0.764 Mev?
Won't it get a little warm?
That looks like a fair bit of energy per nucleon to me.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
https://m.banggood.com/1_5x6mm-Trit-Vials-for-DQG-SPY-DQG-Fa...
https://m.banggood.com/1PCS-2x12mm-Multicolor-Tritium-Vials-...
https://m.banggood.com/3x22_5mm-Trit-Vials-Tritium-Self-lumi...
https://m.banggood.com/1pcs-Trit-Vials-Tritium-Self-luminous...
These might be a little cheaper, faster, safer? But not nearly as likely to give you super powers!
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
They used to be put in the dials (a round thing with ten finger holes used for dialing LOL) of trim phones (first modern British phone). So you could
dial in the dark. They had special evacuation procedures in the event that a box of vials being damaged

Thats way back near the end of the electromachanical days of telephony mid 1960s. When some director (a machine to decode the dialled area code in to
a routing code) centres had hundreds of mostly young female telephonists sat at long rows of switch boards and it was the sixties.
[Edited on 7-2-2018 by wg48]
|
|
|