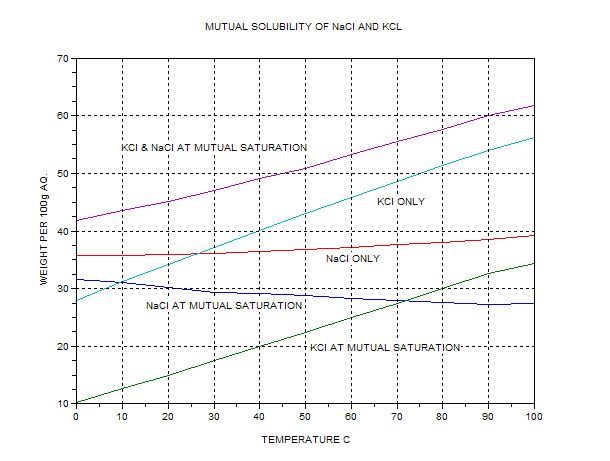
I have recently undergone an experiment to seperate Potassium Chloride from "Low-Salt" which is roughly 66% KCl and 33% NaCl, for use in further
experiments and to create Potassium Chlorate at a later date. I added 336g of Low-Salt to 400ml of boiling water and cooled to 0C. Using the fact
that...
NaCl solubility is relatively constant at between 36-38g/100ml of water.
KCl solubility increases from 28g/100ml at 0C to 56g or so at 100C.
I assumed that i would be able to seperate using differential solubility. 336g of Low Salt contains 112g of NaCl which is well below its saturation
point in water but KCl should precipitate out as i cool it as im using a saturation amount at boiling point. This means that on completing the
experiment i should have been left with 112g of KCl and a 50:50 solution of Kcl and NaCl.
BUT- I have ended up with over 200g of crystals! I hve rigorously microwaved to check i have removed as much moisture as possible (powder anyway) and
dont know whether the powder i have got left is KCl and NaCl or just KCl. From the amount i assume a mixture. If anyone can shed any light on this it
would be great!!
|