| Pages:
1
2 |
Funkerman23
Hazard to Others
  
Posts: 416
Registered: 4-1-2012
Location: Dixie
Member Is Offline
Mood: No Mood
|
|
Seperation of Salts?
Can anyone recommend a method of separating a mixtures of chlorides? For example a mixture of Potassium and sodium chloride? I have looked into using
different solvents( specifically References such as the CRC handbook and ABC Chemie) but I am unsure if sodium chloride is soluble in methanol.. But
to broaden this where should I look for solutions to problems like these?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I am not entirely sure, but directly separating NaCl and KCl is not an easy thing to do.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I attest to Anders . . .I've sifted through huge amounts of data on these compounds in my efforts to separate the KCl from the NaCl that is in the
'Lo-Salt' sold in UK stores. It is 51% KCl and 49% NaCl - just so that they can say 'over half less sodium' etc.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
Those two compounds are industrially separated by fractional crystallization, though I have no idea myself on just how one would go about doing it in
a worthwhile way at home.
If you have small amounts of the mixture just forget it, the cost of both are miniscule compared to the labor cost of separating them.
Do you have a known ratio of the mix? If you do, and one of the constituents are in very low concentration you might just recrystallize a couple of
times and you might get rid of the one, though I suspect those two to co-crystallize..
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
That seems to be the problem, as well as their similar solubilities.
Perhaps it would be an interesting project for the amateur to try to find a simple little way of doing fractional crystallizations at home. Not for
the value of the sodium/potassium chlorides, of course, just to illustrate the point and maybe have a use for it with other chemicals.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Tis no bother a-tall a-tall
See here:
EDIT:
http://www.4shared.com/file/o-Na7wMU/Inorganic_Preparations_...
The book can be had at the above link. Just use any non valid email address to 'log in'.
It easy to do but a total and absoulut PITB. You would need to keep weighing the salts that come out of the system and washing etc etc.
Get muriate of Potash from you local Agri supply store. It's about 60 quid for 50 kg. (about 99%KCl). Recrystallize once for pretty pure stuff.
When separating salts it is the mutual solubility of the salts (in water) that needs to be understood. Mutual Solubility is one of the most BOREING,
least understood and least talked about subjects on planet science.
You can read all about it here: (thanks to Watson Fawkes).
http://www.oxidizing.110mb.com/chlorate/mut_pdf.zip
and more here is you are a gultton for punishement. (Sodium Chlorate + Sodium Chloride)
http://www.oxidizing.110mb.com/chlorate/remove.html
Just to complicate things further, mutual solubility graphs are (probably) graphed after steady state conditions have been reached in the solutions
(or at least some time has been let lapse). You will not get the amounts the the graphs predict crashing out of solution as soon as such and such
temperature is reached. You may have to wait hours for the 'meta stable' (super saturation) states to fade away.
Dann2
[Edited on 7-6-2012 by dann2]
[Edited on 7-6-2012 by dann2]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hear's what the doctor ordered:
If doing this tell us how you got along!
Attachment: kclnaclmut.zip (73kB)
This file has been downloaded 720 times
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Difficult in the very real sense in which it is difficult to:
Add hot water and stir.
Pour off the solution.
Leave to cool.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Chemist must have a sense of imagination (creative thinking) to come up with possible solutions, as well as a good knowledge of chemical reactions.
First, convert both NaCl and KCl together into some NH4Cl by adding ammonia and CO2 (actually, we just created a bigger ionic solution):
NaCl + NH3 + H2CO3 <--> NaHCO3 + NH4Cl (l)
KCl + NH3 + H2CO3 <--> KHCO3 + NH4Cl (l)
This is the so called Solvay process, to quote from Wiki:
"In industrial practice, the reaction is carried out by passing concentrated brine through two towers. In the first, ammonia bubbles up through the
brine and is absorbed by it. In the second, carbon dioxide bubbles up through the ammoniated brine, and sodium bicarbonate (NaHCO3) precipitates out
of the solution. Note that, in a basic solution, NaHCO3 is less water-soluble than sodium chloride. The ammonia (NH3) buffers the solution at a basic
pH; without the ammonia, a hydrochloric acid byproduct would render the solution acidic, and arrest the precipitation."
Link: http://en.wikipedia.org/wiki/Solvay_process
Now, the solubility at 0 C of KHCO3 is a little below (at 22.5) that of NH4Cl (29.4) and lowest for NaHCO3 (7.0). Note, the solubility of KCl (at 28)
is slightly higher than KHCO3 so that the second reaction may move so what to the right (this may not be an issue as the first reaction will move
hopefully largely to the right).
So one could cool the solution to remove the NaHCO3 and add some HCl (this step may be optional as heating alone could reverse the second reaction)
and heat to decompose the NH4Cl and isolate the KCl. In addition, the presence of the other more soluble salts may further aid in precipitating out
(or "salting-out") the Sodium bicarbonate.
Knowing the starting amount (or estimate) of Na and the weight of the NaHCO3 collected may give some info on the success of this path.
[Edited on 10-6-2012 by AJKOER]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
AJKOER: What you forget is the difference between industrial scale processes and small scale lab processes. Many chemicals can be created and isolated
at very low cost in industrial setups. This is done on a multi-tonne scale everyday in factories, making and/or purifying H2SO4, HNO3, NaCl, KCl, KOH,
HCl, NH4NO3 and so on. These chemicals can be made at amazingly low prices (only a few tens of euros per tonne). But these processes are very bad at a
small scale for the following reasons:
- A lot of plant engineering is necessary. The systems are very complicated and can only be built efficiently when the processes they run are allowed
to run at big volumes and for extended periods of time in which huge amounts of the desired chemicals are made.
- The processes use a lot of chemicals for lead-in (start up of chemicals). Before any product can be separated, first a lot of starting materials are
needed. Once the solutions in the plant have reached a certain concentration, addition of more input materials leads to isolable output. When the
plant needs to be stopped temporarily, then the partially processed solutions need to be kept, such that next time, when the process starts up again,
the solutions can be used again for quicker generation of isolable material.
An example of the latter in my own practice at a small scale is the electrolytic production of NaClO3. Initially, it takes a LONG time before any
NaClO3 is separated from the electrolytic cell. At a certain point you get a crop of solid NaClO3. However, next time, when the liquor is kept and
used as a starting material and some fresh salt is added, the separation of NaClO3 is achieved much earlier in the process, because there is no need
to build up a sufficiently high concentration of NaClO3 for precipitation of the solid chemical.
In general one can state that most processes which are very succesful on an industrial scale are not suitable for small scale lab use or home
chemistry.
[Edited on 11-6-12 by woelen]
|
|
|
vampirexevipex
Hazard to Self
 
Posts: 62
Registered: 22-2-2012
Location: Puerto Rico
Member Is Offline
Mood: Happy 
|
|
I had this same problem, all i did was pour the mixture into a solution of sodium chlorate with the same volume of your mixture of potassium and
sodium chloride. The sodium chloride wont react with the chlorate but the potassium chloride will ( KCl + NaClO3 → NaCl + KClO3 ) making
potassium chlorate. After it has reacted the potassium chlorate will precipitate, cool the solution so more potassium chlorate can me made. After
that, just filter the solution.
Reference:
http://chemistry.about.com/od/makechemicalsyourself/a/Potass...
http://www.youtube.com/watch?v=JtxQT7aVDeg&feature=chann...
Why when you add poop to sulfuric acid... a orange precipitate forms?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
My reference to the industrial Solvay process was more for the validation of the chemistry. Here is an actual simple garage chemist approach to
separating these salts based on the chemistry. First some assumptions and calculations, I assume my household ammonia is around 5% NH3 and carbonated
water is about 0.5% CO2 by weight. Hence, the reaction of NaCl with these compounds can be expressed as:
NaCl + [NH3 17.944 H2O] + [CO2 489 H2O] = NaHCO3 + NH4Cl + 505.95 H2O
Also, Lite Salt which claims to have 290 mg Na and 350 mg Potassium per serving, can be expressed as [ NaCl .71KCl ].
Now the KCl reaction is:
KCl + [NH3 17.944 H2O] + [CO2 489 H2O] = KHCO3 + NH4Cl + 505.95 H20
which must be scaled by .71 to simulate a reaction with Lite Salt.
I conducted the following reaction: Lite Salt 22.3 grams (about 10.7 ml); Household ammonia 6.1 ml, Carbonated water 167 ml. The reaction produced a
very fine milky white suspension. I put a fine paper plug in the tube of a large funnel, which successfully (or although slowly) produced a clear
solution upon filtering the cooled solution.
I tested the solution by adding more household ammonia to have it turn milky again. This means that the CO2 solution may have been stronger than I
assumed at 0.5% CO2 (the reported highest CO2 concentration in Seltzer water is about 1%), or the household ammonia was less than 5% (normally between
5% to 10%), or a salting-out is being observed. I added more carbonated water, cooled and filtered again.
My observations (not yet tabulated) is that the above process with dilute solutions may still be able to separate a significant amount of the Sodium
presence assuming primarily a benefit from a salting-out effect (as, based on the solubility of NaHCO3 alone and the amount of dilution noted in the
equations above, hardly any NaHCO3 precipitation is expected).
Clearly, replacing the very diluting soda water with a small amount of Dry Ice (or a CO2 generator) would most likely produce significantly better
results although possibly more costly or less accessible to some.
[Edited on 14-6-2012 by AJKOER]
[Edited on 14-6-2012 by AJKOER]
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
Extracting KCL from a KCl/NaCl mixture.
I originally wrote this some 4 years ago but never posted it, AFAIK.
This very simple process requires a little thought and care to do properly (and scientifically). It is a good example of fractional crystallization.
However, you do need something to weight and measure volume with, and the ability to do simple arithmetic.
Lite Salt (by Morton) contains KCL:NaCl in ratio 35:29 according to the label, plus small amounts of calcium silicate, magnesium carbonate, dextrose,
and potassium iodide. (Don’t get excited about the KI – it’s present at about 50mg/tonne). The other contaminants are insoluble and can be
filtered off, except dextrose, a highly soluble sugar (D-glucose). This can be ignored as we are going to attempt fractional crystallization.
KCl and NaCl both crystallize in the same cubic form but do not form a double salt because the potassium chloride cell unit is much larger than the
sodium salt – the density of KCL is lower than that of NaCl in crystal form (1.99 vs. 2.17), in spite of higher molecular weight.
Beginners often make the mistake that the solubility of a salt does not depend on other salts present. In fact sometimes it is higher, if differing
ions are present, and sometimes less if common ions are present. More advanced amateurs know the common ion equations but they don’t apply to
concentrated solutions with any accuracy due to variation of the activity coefficient. You have to rely on measured results – but they are not
always available. (The graph is my replot from data from BASF, IIRC).
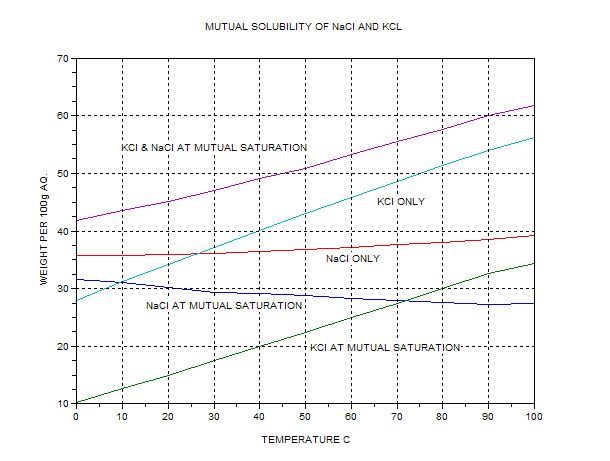
The top curve gives the total combined amount of KCL+NaCl that can exist in solution at various temps. KCl is more soluble at higher temps. The
solubility of NaCl actually decreases if a large amount of KCL is present (common ion effect).
At 100C 62 g combined salt max should dissolve per 100g water, with 34g KCl and 28g NaCl. ‘Lite’ contains a bit more potassium than this. We can
expect this ratio if we saturate 100g (ml) of water with our mixture, which contains 37.7g KCl per 28g NaCl, an excess of about 3.7g if we attempt to
dissolve 65.7g in 100ml at 100C. The excess KCl should not dissolve. Note that the boiling temperature will be above 100C.
So, weigh out 65.7g and dissolve as much as possible at boiling.Filter off rapidly, without too much temperature drop. Collect the salt on the paper
and put aside, label ‘enriched KCl’ or some such, because it certainly isn’t pure yet!
The filtrate should be left to cool and then be refrigerated down to about 0C. According to the graph, 10g of the KCl would be left in solution if the
solution were mutually saturated but now there’s not enough NaCl because now 31g are needed.
KCl left in solution, perhaps 12g. However, there was 34g so we expect about 34-12g=22g of KCl to separate. Filter again and put in the ‘enriched’
pot. So far we have extracted 25.7g out of 37.7g input KCl, i.e. yield about 68% but not very pure.
Now the solution has about 28g NaCl and 12g KCl +100g water (actually some was lost by evaporation and wettingthe filter paper, etc.) – if we
continue, we have to get rid of some NaCl by boiling down the water until the KCl level gets up to the mutually saturated level at 100C.
12g would need 12/34*100 ml ~35 ml of water for solution @100C.; the only way to get this roughly right is to weigh the solution plus the pptd. NaCl
and stop evaporating when the total weight is ~ 12+28+35 = 75g. Cooling this as before would give another 0.68*12g =8.2g KCl after filtering off the
pptd. NaCl hot. This would give about 80% yield. In principle, each step gains 68% of the KCl left but it is rarely worth doing more than two
crystallizations unless you have a valuable substance.
The final step is to purify the KCl which will contain a few percent of NaCl, most of the insoluble contaminants and little of the soluble ones. Use
the KCl only curve to estimate quantities. 56g dissolve at 100C, 28g at 0C per 100ml aq. Thus 50% is extracted per cycle of evaporate and freeze. To
get about 80% needs three cycles of dissolve, cool, evaporate till crystals appear. Or you can evaporate a bit further if the NaCl level is low
enough. It is a good idea to wash the crystals after precipitation with a bit of cold pure water –this washes off the dissolved NaCl at the price of
slightly less, but much purer KCl. You would then have 0.8*0.8*100% of the original KCl in a pure form, 64%
Depending on how carefully you do it, further recrystallization from distilled or de-ionised water may be necessary to obtain KCl that does not show
the Na+ yellow lines in a flame.
Der Alte
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
With respect to my Solvay process separation technique, here is a thorough reference that details the combined solubility of NaHCO3 in the presence of
NaCl and NH4HCO3 (formed in the reaction CO2 + H2O + NH3 <----> NH4HCO3 ). To quote the author:
"The experimental results (Figure 5.4) indicate that for an aqueous solution containing 8%
NaCl, the solubility of NaHCO3 can be reduced to 0.0 g/100g with the addition of about
13wt% ammonium bicarbonate, which can definitely have significant effect on the
possibility of using the Solvay process for reject brine management."
Source: "Reject Brine Management" by Muftah H. El-Naas, United Arab Emirates University, UAE, page 247.
Link:
http://cdn.intechopen.com/pdfs/13761/InTech-Reject_brine_man...
The implication of this comment suggests the possibility of a very good yield (which I am seeing based on the amount of NaHCO3 precipitation) on the
NaCl/KCl separation with the proper concentrations. It also explains my observations as to why increasing the ammonia concentration is desirable as
per the author, to quote from page 245, "This reiterates the importance of ammonia as a catalyst in Reaction (5.1) and the importance of controlling
sodium bicarbonate solubility in the overall process" as the author notes "Ammonia buffers the solution at a basic pH of greater than 9 and hence
allows the precipitation of NaHCO3, which is less water-soluble in basic solution than NaCl." A further comment is: "It is important to note that the
stoichiometric amount of ammonia required by Reaction (5.1) is one mole. However, in a real process excess ammonia may be needed for the reaction to
reach completion. An experimental evaluation of the effect of excess ammonia on the removal of sodium at 20°C (El-Naas et al, 2010) indicated that
the percent removal of sodium increased with increasing the NH3/NaCl ratio, reaching a maximum at 3 as shown in Figure 5.2. Similar experiments with
synthetic brine solution, containing only NaCl in distilled water, in this study and in a previous study (Jibril and Ibrahim, 2001) revealed that the
optimum sodium removal was achieved at a lower molar ratio (NH3/NaCl) of 2. "
I would speculate that household ammonia also contributes to the presence of NH4HCO3 created from the reaction of dissolved CO2 in the tap water used
to produce the household ammonia and together with the small amount of my added Seltzer water fosters NaHCO3 precipitation.
The author in tables 5.1, 5.2 and 5.3 on pages 243 to 244 also details the interesting thermodynamic data for reactions (5.1), (5.5) and (5.6), given
as:
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl (5.1)
NH4OH + CO2 → NH4HCO3 (5.5)
NaCl + NH4HCO3 → NaHCO3 + NH4Cl (5.6)
[Edited on 16-6-2012 by AJKOER]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
AJKOER,
Why?
As I pointed out ( a bit light-heartedly) it's not difficult- you need hot water.
DerAlte has now provided all the data and a detailed method. (Thanks by the way, for doing that).
Why go to the trouble of messing about with ammonia and CO2 (which, if I remember rightly you have to use under pressure)?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Unionised:
My suggested process, after calculating the best amounts of NaCl, NH3 and NH4HCO3 per the reference provided (and perhaps a little experimentation
given the presence of KCl) at 10 C, is exceedingly simple (mix and filter).
And can you beat the author's quoted possible yield (as measured by precipitation of the NaHCO3) approaching an amazing 100%?
Please note that my cruder efforts to date have successfully and quickly produced high Sodium bicarbonate precipitation.
[Edited on 16-6-2012 by AJKOER]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
The joys of mutual solubility
Hello,
I put together a document for to attempt to explain how to read and use simple mutual solubility diagrams or sometimes referred to as phase diagrams.
It is attached in the zip.
Going back to the NaCl/KCl separation:
@DerAlt
Firstly it is probably fair to assume that the minor constituents in the low salt
will not figure in the argument and can be ignored. I presume you have done that.
One figure I can't understand is:
".......water with our mixture, which contains 37.7g KCl per 28g NaCl, an excess ..."
Are you referring to the ratio of KCl to NaCl in Lite-Salt?
If so it should be 33.79 grams KCl per 28 grams NaCl. (assuming Lite-Salt is 35/29).
When you add the Lite-Salt to the 100g water at 100°C and wait and stir for everyting
that is going to dissolve to actually dissolve. (No evaporation allowed.)
If there is a very small amount of Solid over it will be pure NaCl. If there is a larger amount of solid left over it will be mixed solid
(mixture of NaCl and KCl) very slightly enriched with NaCl.
That's my understanding looking at a mutual solubility diagram. I cannot see how you would have
Lite-Salt inriched with KCl on the bottom.
Anyhow I don't disagree with the overall method though I cannot quite get my head around how you
obtain the argument from the graphs that you are using!
If I were to take a stab at the problem I would have to use the mutual solubility diagram
(blow by blow method). See the attached gif.
I would take an slight excess of Lite-Salt (about 66 grams, use 70 grams to be sure)
and dissolve all that would dissolve in 100ml water at 100°C to make a solution
saturated with both NaCl and KCl. If using Lo-Salt use about 85 grams.
We will have a system at point Q. Filter or decant
this hot solution. This will get rid of any insoluble matter together with the excess
KCl and NaCl on the bottom.
To this hot (100°C) solution I would add a few ml hot water to make sure no NaCl will
come out of solution as you are very near the NaCl 'field'. The hot solution is represented
by the black dot on the knee of the 100°C curve.
This is a system containing 22% KCl, 17% NaCl and 61% water. That's 100
grams water + 36 grams KCl + 27.9 grams NaCl = 164 grams total weight in system. By adding
a few ml hot water (keeping all at 100°C so that no NaCl will drop out before you make
the addition) we will have a system represented by point M.
Then cool (slowly to get big crystals) to zero. It is important that you do not let any
water evaporate out as you cool. You could go down to -10°C or so? to get more KCl
out but I have no data for below zero.
According to the mutual solubility diagram (an interpreted by Dann2!)you will get around
13.3% of the total system weight coming out of solution. Thats around 22 grams of
pure KCl as you are in the pure KCl field through out the cooling time.
At this stage you remove the KCl and you are left with a solution that is 142 grams in weigh.
You can now start boiling off water down to a weight in the region of 82 grams
(60 grams boiled off) about 42% of system weigh. FILTER HOT and add a few ml of hot water
The hot liquid you now have is in the region of the starting point
Cool and some more KCl will come out.
It probably best to just go with the first crop of KCl and leave at that.
It may be much more convienent to work at 80°C instead of boiling as it can be
dangerous. Not impossible to handle by any means but accidents can happen.
If making Chlorae
(which most people who do this may be doing) it's MUCH easier to seperate the K from the Na
salts at the Chlorate stage.
Attachment: Mut_Sol.zip (209kB)
This file has been downloaded 955 times 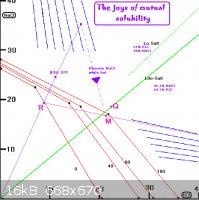
[Edited on 25-6-2012 by dann2]
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
In the attempt of cheapen the process of purification through the chlorate route, as in the case of commonly already pretty pure KOH which contains up
to 16% Na, is it plausible to retrieve the chlorate and recycle it thus saving the hypochlorite needed to produce the chlorate?
3 NaClO --> heat --> NaClO3 + 2 NaCl (expensive part)
purification:
NaClO3 + KCl (85%) + NaCl (15%) --> KClO3 (ppt) + NaCl(aq)
Ion exchange
C4H6O6 + NH4OH → NH4C4H5O6 + H2O
H4C4H5O6 + KClO3 → KC4H5O6 + NH4ClO3
Thus the potassium has been purified whilst NH4ClO3 can be used to prepare KClO3 again.
[Edited on 8-3-2012 by Poppy]
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
You could perhaps use the sulfate ion to precipitate Na2SO4.
Which one are you after? The sodium salt or potassium?
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
I have another idea. Because of the common ion effect, we could try adding an extremely soluble sodium salt (sodium hydroxide/acetate comes to mind)
to some water, and add the mix of NaCl and KCl into the solution. Only a small amount of NaCl will dissolve, because there is already an excess of Na+
on the dissociation equilibrium. In contrast, this will cause a comparatively larger amount of KCl to dissolve, because only a small amount of NaCl
dissolved, so the common ion effect would not be as great. Stir for a few minutes, then take the solution and crystallize out KCl.
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
weiming1998:
You can solve how to get KCl/ NaCl mix out of their crystal shape selectively. Because the front of dislocation the crystal lattice will end on to
hold both ions and the precedure won't be successful to any useful extent. You must heat and dissolve both and then carefully add nucleation centers
for the NaCl material that will come out.
Back to the chlorate method I guess its in favor to worth the try theres whole processes involving recovery of both tartaric acid and chlorate by
means of sulfuric acid and calcium chloride, these alter all cheaper IMHO
Thus follows the handsome half-equations:
KClO3(aq) -> K+(ppt) + ClO3(-)(aq)
|
|
|
Zirconium
Harmless

Posts: 1
Registered: 16-4-2013
Member Is Offline
Mood: No Mood
|
|
I have recently undergone an experiment to seperate Potassium Chloride from "Low-Salt" which is roughly 66% KCl and 33% NaCl, for use in further
experiments and to create Potassium Chlorate at a later date. I added 336g of Low-Salt to 400ml of boiling water and cooled to 0C. Using the fact
that...
NaCl solubility is relatively constant at between 36-38g/100ml of water.
KCl solubility increases from 28g/100ml at 0C to 56g or so at 100C.
I assumed that i would be able to seperate using differential solubility. 336g of Low Salt contains 112g of NaCl which is well below its saturation
point in water but KCl should precipitate out as i cool it as im using a saturation amount at boiling point. This means that on completing the
experiment i should have been left with 112g of KCl and a 50:50 solution of Kcl and NaCl.
BUT- I have ended up with over 200g of crystals! I hve rigorously microwaved to check i have removed as much moisture as possible (powder anyway) and
dont know whether the powder i have got left is KCl and NaCl or just KCl. From the amount i assume a mixture. If anyone can shed any light on this it
would be great!!

|
|
|
platedish29
Hazard to Self
 
Posts: 76
Registered: 2-9-2012
Member Is Offline
Mood: absorbing CO2
|
|
Do a flame test. Although it's not worth anything since Na contaminations tend to shade the flame yellowish even at trace ammounts...;
Do a gravimetric test, if you have a will fit scale for this;
Do a solubility test;
Recrystalize.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Quote: Originally posted by Zirconium  | I have recently undergone an experiment to seperate Potassium Chloride from "Low-Salt" which is roughly 66% KCl and 33% NaCl, for use in further
experiments and to create Potassium Chlorate at a later date. I added 336g of Low-Salt to 400ml of boiling water and cooled to 0C. Using the fact
that...
NaCl solubility is relatively constant at between 36-38g/100ml of water.
KCl solubility increases from 28g/100ml at 0C to 56g or so at 100C.
I assumed that i would be able to seperate using differential solubility. 336g of Low Salt contains 112g of NaCl which is well below its saturation
point in water but KCl should precipitate out as i cool it as im using a saturation amount at boiling point. This means that on completing the
experiment i should have been left with 112g of KCl and a 50:50 solution of Kcl and NaCl.
BUT- I have ended up with over 200g of crystals! I hve rigorously microwaved to check i have removed as much moisture as possible (powder anyway) and
dont know whether the powder i have got left is KCl and NaCl or just KCl. From the amount i assume a mixture. If anyone can shed any light on this it
would be great!!
|
How did you come up with 112 g of KCl?
336 g * 0.66 = 222 g of KCl
You also have 114 g of NaCl
In principal, the 114 g of NaCl should all stay dissolved but the fact that you have NaCl dissolved effects the solubility of the KCl.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
In practice, you'll have to perform several such recrystallizations to effect full separation. Then one or two nice recrystallizations before the
sodium orange flame color actually goes away.
Oddly, I've made "orange-free" potassium chlorate from sodium chlorate, but the solubility difference is much more dramatic.
Tim
|
|
|
| Pages:
1
2 |