
and not an alcohol (other than one oxidizable to give a ketone, such as isopropanol (which would of course give acetone)). When ethanol is oxidised, acetaldehyde is produced and that is then usually further oxidized to acetic acid, neither of which are ketones (but they are all carbonyl compounds).
Also, acetone is pretty widely available from numerous sources - notably from hardware stores as a solvent or from nail polish remover, but alas I cannot say whether the same is true for Australia. It is usually fairly cheap, and, if you can't get it, then why not either a) look for isopropanol, which may be more widely available in your area, or b) buy it online?
[Edited on 24-4-2012 by Hexavalent]

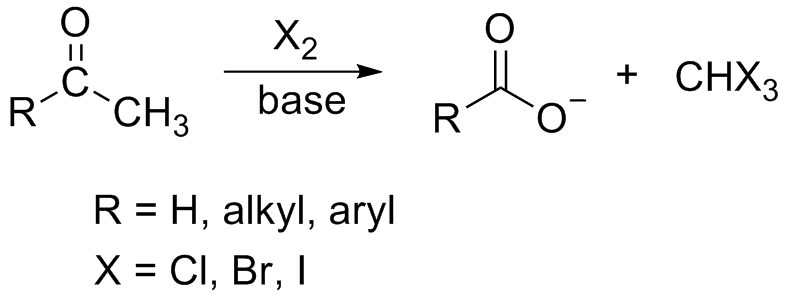
 . R can be equal to H.
. R can be equal to H.