weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
Haloform reaction with ethanol-failure?
A few days ago, I have been thinking about utilizing the haloform reaction to make some chloroform that might be useful as a solvent. Acetone is
expensive, and I don't want to use that, so I am trying to make it through a haloform reaction with ethanol instead.
What I tried proceeds as:
First 50mls of EtOH is poured into a narrow-necked bottle. An unmeasured amount of Ca(ClO)2 is added into the ethanol through a funnel. A plastic
stopper with a rubber tube through it is immediately capped onto the bottle. It is then placed in a cooling bath (later changed to heating) and the
off gases (which occured regardless if the solution is chilled or not) are bubbled through water. The solution is then filtered- and found to be
miscible with water, which shouldn't happen considering chloroform is immiscible with water.
After countless attempts, I could not get the chloroform to precipitate. It remains dispersed in the solution and gives it a foggy, white, translucent
appearance, even if I added salt to the solution. Distillation of the product produced a mix of vapours and liquid, so I have to lead it through
water for it to all liquify, and once I lead that through water, it fogs up the solution and refuse to precipitate again! The closest attempt was when
I added some water to ethanol. It stated precipitating a bit, but after 2mls of it came over, it started the fogging again!
I found countless references on the internet (and on this forum), but none helped with the soluble chloroform problem. I apologise if I have not
searched more deeply.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | An unmeasured amount |
No scale? Even then you should try to estimate the amount added. It would be difficult to add enough solid Ca(OCl)2 to turn all the ethanol into
chloroform, and if you could it would probably be dangerous.
Anyway, the one time I tried to use ethanol for the haloform reaction (using sodium hypochlorite) I got nowhere. Either the initial oxidation of
ethanol to acetaldehyde is slow or else acetaldehyde reacts more slowly than acetone for this purpose (I would guess the former). You could try to
track down some description of the original 19th century experiments on this reaction to see what conditions they used in order to perform the
reaction with ethanol.
The less you bet, the more you lose when you win.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Organic chemistry is not my speciality but I thought that the haloform had to involve a methyl ketone;
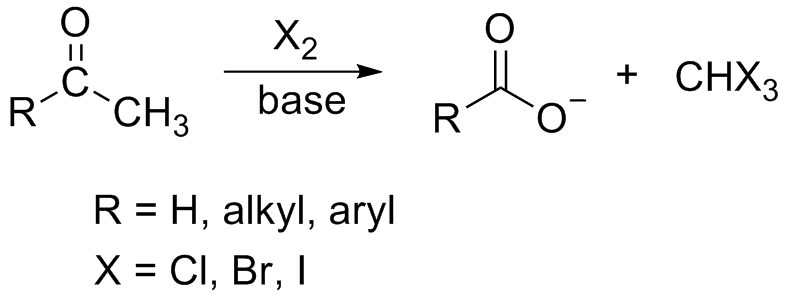
and not an alcohol (other than one oxidizable to give a ketone, such as isopropanol (which would of course give acetone)). When ethanol is oxidised,
acetaldehyde is produced and that is then usually further oxidized to acetic acid, neither of which are ketones (but they are all carbonyl compounds).
Also, acetone is pretty widely available from numerous sources - notably from hardware stores as a solvent or from nail polish remover, but alas I
cannot say whether the same is true for Australia. It is usually fairly cheap, and, if you can't get it, then why not either a) look for isopropanol,
which may be more widely available in your area, or b) buy it online?
[Edited on 24-4-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Look at your own reply  . R can be equal to H. . R can be equal to H.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
Quote: Originally posted by Hexavalent  | Organic chemistry is not my speciality but I thought that the haloform had to involve a methyl ketone;

and not an alcohol (other than one oxidizable to give a ketone, such as isopropanol (which would of course give acetone)). When ethanol is oxidised,
acetaldehyde is produced and that is then usually further oxidized to acetic acid, neither of which are ketones (but they are all carbonyl compounds).
Also, acetone is pretty widely available from numerous sources - notably from hardware stores as a solvent or from nail polish remover, but alas I
cannot say whether the same is true for Australia. It is usually fairly cheap, and, if you can't get it, then why not either a) look for isopropanol,
which may be more widely available in your area, or b) buy it online?
[Edited on 24-4-2012 by Hexavalent] |
Yes, if R is H, then the haloform reaction can proceed normally, producing calcium formate instead of the acetate.
I can buy acetone and isopropanol, but the 4L methylated spirits is cheaper than acetone (1L for $10 compared to the price of $12 for 4L) or the
isopropanol. I was just looking for a cheaper way.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by weiming1998  |
What I tried proceeds as:
First 50mls of EtOH is poured into a narrow-necked bottle. An unmeasured amount of Ca(ClO)2 is added into the ethanol through a funnel. A plastic
stopper with a rubber tube through it is immediately capped onto the bottle. It is then placed in a cooling bath (later changed to heating) and the
off gases (which occured regardless if the solution is chilled or not) are bubbled through water. The solution is then filtered- and found to be
miscible with water, which shouldn't happen considering chloroform is immiscible with water.
|
No water in the reaction? The haloform reaction has to run in aqueous solution, not in ethanol only. Try adding 100ml water per 20g calcium
hypochlorite.
That, and the oxidation of ethanol to acetaldehyde requires an extra mole of hypochlorite, so you get less chloroform from your hypochlorite than when
using chloroform.
Also, the method with ethanol never worked properly for me as well.
|
|
|