Difference between revisions of "Octyl acetate"
From Sciencemadness Wiki
(Created page with "300px '''Octyl acetate''' is an ester which is commonly found in citrus fruits. ==Properties== ===Chemical=== Octyl acetate can...") |
|||
| Line 4: | Line 4: | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Octyl acetate can hydrolyze to 1-octanol and acetic acid. It can also act as a solvent. | + | Octyl acetate can hydrolyze to [[1-octanol]] and [[acetic acid]]. It can also act as a solvent. |
===Physical=== | ===Physical=== | ||
| − | Octyl acetate is insoluble in water but soluble in ethanol, [[1-octanol]] and ether. It has a fruity smell. Octyl acetate has a wide liquid range. It melts at -38°C and boils at 211°C. | + | Octyl acetate is insoluble in water but soluble in ethanol, [[1-octanol]] and [[diethyl ether]]. It has a fruity smell. Octyl acetate has a wide liquid range. It melts at -38°C and boils at 211°C. |
==Availability== | ==Availability== | ||
Revision as of 14:57, 3 August 2015
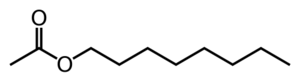
Octyl acetate is an ester which is commonly found in citrus fruits.
Contents
Properties
Chemical
Octyl acetate can hydrolyze to 1-octanol and acetic acid. It can also act as a solvent.
Physical
Octyl acetate is insoluble in water but soluble in ethanol, 1-octanol and diethyl ether. It has a fruity smell. Octyl acetate has a wide liquid range. It melts at -38°C and boils at 211°C.
Availability
Octyl acetate may be extracted from oranges or grapefruits.
Preparation
As octyl acetate is an ester of 1-octanol and acetic acid, it can be synthesized by adding the two together in dehydrating conditions, though this is difficult.
Projects
- Extract octyl acetate from oranges or grapefruits
Handling
Safety
Octyl acetate is not particularly toxic, but do not ingest lab-grade material.
Storage
Should be stored in closed bottles, away from any flame source.
Disposal
Octyl acetate can be safely burned.