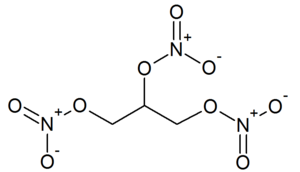
Nitroglycerin
| |
| Names | |
|---|---|
| Preferred IUPAC name
Propane-1,2,3-triyl trinitrate | |
| Other names
1,2,3-Tris(nitrooxy)propane
Glyceryl trinitrate Nitro-dur Nitroglycerol Nitrostat Trinitroglycerin Trinitroglycerine | |
| Properties | |
| C3H5N3O6 | |
| Molar mass | 227.09 g/mol |
| Appearance | Colorless to pale-yellow liquid |
| Odor | Odorless |
| Density | 1.6 g/cm3 (at 15 °C) |
| Melting point | 14 °C (57 °F; 287 K) |
| Boiling point | 50 °C (122 °F; 323 K) (explodes) |
| 0.138 g/100 ml (at 20 °C) | |
| Solubility | Miscible with 1,2-dibromoethane, glacial acetic acid, dichloroethylene, ethyl acetate, nitrobenzene, pyridine Slightly soluble in carbon disulfide, ethanol, methanol Poorly soluble in glycerol, liq. petrolatum, oleic acid, petroleum ether, toluene |
| Solubility in carbon disulfide | 0.833 g/100 ml |
| Solubility in ethanol | 25 g/100 ml |
| Solubility in methanol | 5.55 g/100 ml |
| Vapor pressure | 0.0003 mmHg (at 20 °C) |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
−370 kJ/mol |
| Hazards | |
| Safety data sheet | CPCB |
| Related compounds | |
| Related compounds
|
Ethylene glycol dinitrate 1,2,4-Butanetriol trinitrate Erythritol tetranitrate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitroglycerin (NG), more correctly called glycerol trinitrate, is the nitrate ester of glycerol. It is one of the most commonly used explosives, found in dynamites, blasting gelatine and smokeless powders.
Contents
[hide]Properties
Physical
Nitroglycerin is a dense (1.59 g/cm3), oily, colourless liquid with freezing point 14 °C. It is a solvent for nitrocellulose, and the resulting gel is known as blasting gelatine (blasting jelly).
Explosive
Nitroglycerin is a powerful explosive, similar to ethylene glycol dinitrate. It is highly sensitive to impact, but incomplete detonation result from everything but a strong initiation. This causes its lead block test value to be dependent on the strength of initiation:
- detonator No.1 ... 190 cm3
- detonator No.2 ... 225 cm3
- detonator No.6 ... 460 cm3
- detonator No.8 ... 590 cm3
Nitroglycerin will not detonate if ignited with an external flame, but if poured on a hot surface with its temperature >200 °C, it will rapidly decompose and almost immediately detonate.[1]
Availability
Nitroglycerin pills contain a very small amount of nitroglycerin, impractical for extraction and use (though some types of pills will burn if ignited).
The classic dynamite contains NG mixed with kieselgur (diatomaceous earth), to make it stable. Most smokeless powders contain nitro. Extracting the nitroglycerin from these products however, is impractical and dangerous for large quantities.
Preparation
Nitroglycerin is prepared by the nitration of glycerol using a concentrated mixture of nitric and sulfuric acids in an ice bath, at very low temperatures. The information in this article is not enough to attempt such a synthesis though, as the risks are very high with this compound.
Just an idea how powerful a small amount of nitroglycerin is, here is a good demonstration of what 1 ounce (28 g) of pure nitroglycerin does to a heavy steel plate.
Projects
- Dynamite
- Smokeless powders
- Various propellents
Handling
Safety
Nitroglycerin is a dangerously sensitive and extremely powerful explosive. Improperly neutralized it is even more sensitive. It is also toxic by skin contact and inhalation. As a strong vasodilator, it results in severe headaches, though it is used medicinally in very low concentrations.
Storage
Never store liquid nitroglycerin, even for short periods!
Dynamite, which consists of nitroglycerin mixed with diatomite, is much more stable, though over time the dynamite will "sweat" or "weep" its nitroglycerin, which can then pool in the bottom of the box or storage area and can explode.
Disposal
Nitroglycerin can be neutralized by carefully diluting it first in a solvent, then slowly adding it dropwise in a diluted solution of sodium hydroxide or being exposed to sunlight for a while, also diluted. Concentrated stuff may explode.
References
Chemistry and Technology of Explosives - Volume II, first edition, 1965.