Nickel hydrazine nitrate
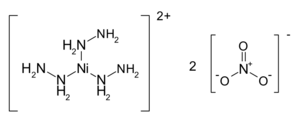 Structure of the NHN coordination compound
| |
| Names | |
|---|---|
| IUPAC name
Nickel hydrazine nitrate
| |
| Other names
Nickel hydrazinium nitrate
| |
| Properties | |
| NiH12N8O6 [Ni(N2H4)3](NO3)2 | |
| Molar mass | 278.69 g/mol |
| Appearance | Purple solid |
| Odor | Odorless |
| Density | 2.129 g/cm3 |
| Insoluble | |
| Solubility | Insoluble in alcohols, ether |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
-449 kJ/mol |
| Hazards | |
| Safety data sheet | None |
| Related compounds | |
| Related compounds
|
Hydrazine nitrate Nickel(II) nitrate Nickel hydrazine perchlorate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nickel hydrazine nitrate (NHN), is an energetic coordination compound having explosive properties in between that of primary and secondary, with the chemical formula [Ni(N2H4)3](NO3)2.
Contents
[hide]Properties
Chemical
Nickel hydrazine nitrate decomposes violently, releasing nickel powder and combustion gases.
Physical
Nickel hydrazine nitrate is a purple solid, practically soluble in water.
Explosive
NHN straddles the line between primary and secondary. Because of this it is a relatively safe explosive to work with having much less sensitivity to shock and friction (16.0 N) than Lead Azide (0.1N). Its detonation velocity is estimated around 7,000 m/s.[1]
Availability
Nickel hydrazine nitrate is not sold by chemical suppliers and has to be made.
Preparation
Nickel(II) nitrate is added to an aqueous solution of hydrazine (hydrazine hydrate), which is kept heated to 65 °C, in order to allow hot filtration. The solution should be 'hot filtered' at 65 °C. Letting it cool down first and then filtering will give an inconsistent product. Approximately 0.1-1% of the total system by weight of dextrin may be added to increase the bulk density from 0.9 mg/cm3 to 1.2 mg/cm3. Successfully produced NHN will have a talcum powder-like consistency when dried without the need to crush the NHN. Unsuccessfully produced NHN will have the aspect of hard chunks that need to be crushed and then maintain a consistency of grains of sand. The latter is the result of mixing at a lower temperature and using alcohol instead of water as the mixing solution.[2][3][4]
Projects
- Blasting cap
Handling
Safety
Nickel nitrate contains nickel and hydrazine, both which are harmful.
Storage
NHN should be kept in small amounts in small containers. While it's stable for long periods of time, it's best to use it as soon as possible.
Disposal
Can be easily neutralized by safely detonating in a safe or remote area.
References
- Jump up ↑ http://www.chemikinternational.com/pdf/2011/01_2011/chemik_2011_65_1_24-27.pdf
- Jump up ↑ https://www.sciencedirect.com/science/article/pii/S0304389402002479?via%3Dihub
- Jump up ↑ https://onlinelibrary.wiley.com/doi/abs/10.1002/prep.19970220604
- Jump up ↑ http://nopr.niscair.res.in/handle/123456789/5478