Haematoxylin
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
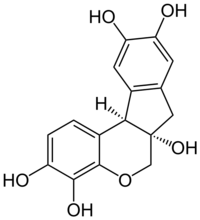
| |
| Names | |
|---|---|
| IUPAC name
7,11b-Dihydroindeno[2,1-c]chromene-3,4,6a,9,10(6H)-pentol
| |
| Properties | |
| C16H14O6 | |
| Molar mass | 302.28 g/mol |
| Appearance | White to yellowish solid |
| Melting point | 148–151[1] °C (298–304 °F; 421–424 K) (decomposes) |
| Boiling point | Decomposes |
| Slightly soluble | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Haematoxylin also called hematoxylin, natural black 1 or C.I. 75290 is a compound extracted from the heartwood of the logwood tree, where it exists as the dextro isomer, (+)-Hematoxylin.
Contents
[hide]Properties
Chemical
Hematoxylin is a basic / positive compound that binds to and forms salts with acidic, or basophilic, compounds containing negative charges (such as DNA and RNA which are acidic/negative because the nucleic acid building blocks that come off the phosphate backbone are negatively charged) and stains them dark blue or violet. Haematoxylin and eosin together make up haematoxylin and eosin stain, one of the most commonly used stains in histology. This type of stain is a permanent stain as opposed to temporary stains (e.g. iodine solution in KI).
Physical
Haematoxylin is a white or slightly yellow solid compound.
Availability
Can be extracted from the heartwood of Haematoxylon campechianum.
Some hair care products are said to contain this compound.
Preparation
It's much cheaper to extract or buy this compound, than to make it yourself.
Projects
- Dye and biological stain
Handling
Safety
There is little data about the toxicity of this compound.
Storage
In closed bottles.
Disposal
No special disposal is required.
References
- Jump up ↑ Masuda; Ohtani; Mizutani; Ogawa; Kasai; Tanaka; Chemical and Pharmaceutical Bulletin; vol. 39; nb. 6; (1991); p. 1382 - 1384