Caffeine
 OTC powdered caffeine
| |
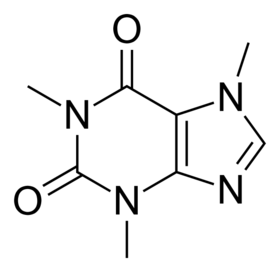 Structure of caffeine
| |
| Names | |
|---|---|
| IUPAC name
1,3,7-Trimethylpurine-2,6-dione
| |
| Other names
1,3,7-Trimethylxanthine
Guaranine Methyltheobromine Theine | |
| Properties | |
| C8H10N4O2 | |
| Molar mass | 194.19 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.23 g/cm3 |
| Melting point | 235–238 °C (455–460 °F; 508–511 K) |
| Boiling point | 178 °C (352 °F; 451 K) (sublimes) |
| 2.17 g/100 ml (25 °C) 20.0 g/100 ml (80 °C) 66.6 g/100 ml (100 °C)[1] | |
| Solubility | Soluble in chloroform, ethyl acetate, pyridine, pyrrole, THF Slightly soluble in benzene, ethanol, petroleum ether |
| Solubility in acetone | 2 g/100 ml[2] |
| Solubility in benzene | 1 g/100 ml |
| Solubility in chloroform | 18.2 g/100 ml |
| Solubility in diethyl ether | 0.2 g/100 ml |
| Solubility in ethanol | 1.5 g/100 ml |
| Vapor pressure | 9·10-7 mm Hg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
127 mg/kg (mouse, oral) 367.7 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Theobromine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Caffeine is an organic chemical compound, an alkaloid, of the methylxanthine class. It is widely used as central nervous system (CNS) stimulant, in the form of coffee.
Contents
[hide]Properties
Chemical
Caffeine reacts with acids to give salts of caffeine. Reaction with citric acid will give caffeine citrate.
Physical
Caffeine is an odorless white solid, slightly soluble in water, though its solubility increases with temperature and the addition of an acid. It shows good solubility in dichloromethane and chloroform.
Availability
Caffeine is available as pills in most pharmacies and drugstores.
Caffeine is more readily available in the form of coffee.
Preparation and isolation
It's not economical to make caffeine from precursors. Extracting it from readily available sources is the only economical way to get useful amounts.
Extraction from caffeine pills
Crush a desired amount of caffeine pills in a mortar until they are turned into a fine powder. The fine powder is then transferred into a flask or beaker. To the powder from the beaker, a volume of dichloromethane is added and the suspensions is stirred for a few minutes. The suspension is left to decant, then filtered using a fritted funnel (or funnel with a proper filter paper), to remove the solid. The filtrate is transferred in a round bottom flask, which is connected to a distillation setup or a rotary evaporator to remove and recover the DCM via distillation. After all the DCM has been removed from the flask, it is transferred in another beaker, where hot water is added until it completely dissolves. The solution is cooled to recrystallize the caffeine. The crude caffeine crystals are filtered using a filter paper or fritted funnel and dried. The crude caffeine can be further purified via sublimation. A good procedure can be found here.
Extraction from coffee
Similar to above, though the yields are much lower. NileRed made a video on this process.
Projects
- Make caffeine salts
- Compound collection
Handling
Safety
Caffeine, as found in coffee and tea, has stimulant effects when consumed. Side effects include elevated blood pressure, restlessness, insomnia, headaches. Caffeine overdose can result in a state of central nervous system over-stimulation called caffeine intoxication.
Storage
Caffeine should be kept in plastic or glass bottles, away from light and corrosive fumes.
Disposal
No special disposal is required.
References
- Jump up ↑ https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/Product_Information_Sheet/c0750pis.pdf
- Jump up ↑ O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 289