Benzocaine
| |
| Names | |
|---|---|
| IUPAC name
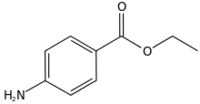
Ethyl 4-aminobenzoate
| |
| Systematic IUPAC name
Ethyl 4-aminobenzoate | |
| Other names
Anbesol
Orajel Cepacol | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C9H11NO2 | |
| Molar mass | 165.189 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.17g/cm3 |
| Melting point | 89 °C (192 °F; 362 K) |
| Boiling point | 310 °C (590 °F; 583 K) |
| Slightly soluble | |
| Solubility | Very soluble in chloroform, diethyl ether, ethanol Soluble in diluted acids |
| Hazards | |
| Safety data sheet | ScienceLab |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzocaine is a topical anesthetic that helps to numb the pain and itching. It's used as a topical pain reliever or in cough drops. Benzocaine combined with antipyrine forms A/B otic drops to relieve ear pain and remove earwax.
Contents
[hide]Properties
Chemical
Benzocaine is the ethyl ester of p-aminobenzoic acid (PABA). It can be prepared from PABA and ethanol by Fischer esterification or via the reduction of ethyl p-nitrobenzoate.
Physical
Benzocaine is sparingly soluble in water; it is more soluble in dilute acids and very soluble in ethanol, chloroform and ethyl ether.
Availability
Benzocaine is sold in various drugstores.
Preparation
Benzocaine can be prepared by esterfication using 4-aminobenzoic acid and ethanol. It can also be prepared by reduction of ethyl 4-nitrobenzoate to the amine.
In industrial practice, the reducing agent is usually iron and water in the presence of a little acid.
Projects
- Local anesthetic
Handling
Safety
Do not use benzocaine topical if you have ever had methemoglobinemia in the past.
Storage
Benzocaine should be stored in closed bottles, away from moisture and air, if stored for long periods of time.
Disposal
Benzocaine can be either oxidized with Fenton's reagent or poured down the drain, depending on the amount of waste you intend to dispose.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Aromatic compounds
- Esters
- Amines
- Materials unstable in basic solution
- Materials unstable in acidic solution
- Readily available chemicals