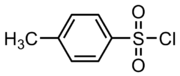
4-Toluenesulfonyl chloride
| |
| Names | |
|---|---|
| IUPAC name
4-Methylbenzene-1-sulfonyl chloride
| |
| Other names
4-Methylbenzenesulfonyl chloride
4-Toluenesulfonyl chloride p-Toluenesulfonyl chloride TosCl Tosyl chloride p-TsCl Toluene-p-sulfonyl chloride TsCl | |
| Properties | |
| C7H7ClO2S CH3C6H4SO2Cl | |
| Molar mass | 190.65 g/mol |
| Appearance | White solid |
| Density | 1.3 g/cm3 |
| Melting point | 69–71 °C (156–160 °F; 342–344 K) |
| Boiling point | 220 °C (428 °F; 493 K) (decomposes) 146 °C (295 °F; 419 K) (at 15 mmHg) |
| Reacts | |
| Solubility | Reacts with alcohols, amines Soluble in benzene, chloroform, diethyl ether, THF, toluene |
| Vapor pressure | 0.0012 mmHg |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 128 °C (262 °F; 401 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tosyl chloride or 4-Toluenesulfonyl chloride is an organic compound with the formula CH3C6H4SO2Cl. It is often abbreviated TsCl or TosCl.
Contents
[hide]Properties
Chemical
Tosyl chloride converts alcohols into the corresponding toluenesulfonate esters or tosylates:
- CH3C6H4SO2Cl + ROH → CH3C6H4SO2OR + HCl
Likewise, TsCl is used to prepare sulfonamides from amines:
- CH3C6H4SO2Cl + R2NH → CH3C6H4SO2NR2 + HCl
The preparation of tosyl esters and amides are conducted in the presence of a base, which absorbs hydrogen chloride. The selection of the base is often crucial to the efficiency of tosylation. Typical bases include pyridine and triethylamine.
Physical
Tosyl chloride is a colorless solid, which reacts with water and alcohols, but dissolves in ethers.
Availability
Can be bought from chemical suppliers.
Preparation
Tosyl chloride can be synthesized via the chlorosulfonation of toluene, using sulfuryl chloride:[1]
- CH3C6H5 + SO2Cl2 → CH3C6H4SO2Cl + HCl
Alternatively, it can be obtained in two steps, by reaction of conc. sulfuric acid with toluene to form toluenesulfonic acid, which is then chlorinated with phosphorus pentachloride to tosyl chloride.[2]
Projects
- Make sulfonamides
- Protecting group for amines
- Compound collecting
Handling
Safety
Tosyl chloride readily reacts with water to form HCl, which is corrosive. Proper protection must be worn when handling this compound.
Storage
Should be kept in dry airtight containers, preferably under inert gas if possible.
Disposal
Should be neutralized with a diluted NaOH solution then destroyed with an oxidizing solution, such as Fenton's reagent.
References
- Jump up ↑ https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_507
- Jump up ↑ https://prepchem.com/synthesis-of-4-toluenesulfonyl-chloride