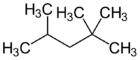
2,2,4-Trimethylpentane
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,2,4-Trimethylpentane
| |||
| Other names
224TMP
Iso-octane Isobutyltrimethylmethane | |||
| Properties | |||
| C8H18 | |||
| Molar mass | 114.23 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | Gasoline-like | ||
| Density | 0.692 g/cm3 | ||
| Melting point | −107.38 °C (−161.28 °F; 165.77 K) | ||
| Boiling point | 99.30 °C (210.74 °F; 372.45 K) | ||
| Almost insoluble in water | |||
| Solubility | Miscible with acetone, benzene, carbon disulfide, carbon tetrachloride, chloroform, dichloromethane, diethyl ether, dimethylformamide, ethanol, heptane, toluene, xylene | ||
| Vapor pressure | 5.5 kPa (at 21 °C) | ||
| Thermochemistry | |||
| Std molar
entropy (S |
328.03 J·K−1·mol−1 | ||
| Std enthalpy of
formation (ΔfH |
−260.6 to −258.0 kJ·mol−1 | ||
| Hazards | |||
| Safety data sheet | Sigma-Aldrich | ||
| Flash point | −12 °C (10 °F; 261 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2,2,4-Trimethylpentane, also known as iso-octane or simply 224TMP, is an organic compound, an isomer of octane (C8H18).
This particular isomer is an important component of gasoline and the standard 100 point on the octane rating scale (with 0 point being heptane).
Contents
[hide]Properties
Chemical
2,2,4-Trimethylpentane is exceedingly flammable in the presence of oxygen, and when ignited will burn to release carbon dioxide and water vapors.
- C8H18 + 25/2 O2 → 8 CO2 + 9 H2O
Physical
2,2,4-Trimethylpentane is an odorless colorless liquid, insoluble in water but miscible with many organic solvents. It lighter than water, with a density of 0.692 g/l. 224TMP has a boiling point of 99.1 °C and a melting point of −107.44 °C. It is very flammable, with a flash point of -12 °C and an autoignition temperature of 396 °C.
Availability
2,2,4-Trimethylpentane is available in high octane gasoline, and can be extracted via fractional distillation or rectification, depending on the composition of gasoline.
Preparation
Industrially it is produced by distillation of petroleum.
Another route involves the dimerization of isobutylene, using an Amberlyst catalyst, method that produces a mixture of iso-octenes. This mixture is hydrogenated to yield 2,2,4-trimethylpentane.
Projects
- Organic extractions
- Pepsin extraction[1]
Handling
Safety
2,2,4-Trimethylpentane poses little toxicity, though its vapors are irritant to the nose, lungs and eyes.
Storage
224TMP should be stored in closed glass or PE bottles away from any heat source.
Disposal
2,2,4-Trimethylpentane can be safely burned.