| Pages:
1
2 |
0010110
Harmless

Posts: 6
Registered: 16-7-2006
Member Is Offline
Mood: No Mood
|
|
Mg metal from MgO
I have a pretty large quanity of MgO and I was wondering how could a go about making it into Mg metal. I was thinking of trying to heat MgO in
distilling flask then distill with a liebig condensor. Does it sound like it will work. i have Like 800 grams of MgO thats a nice bit of Mg metal.
What would be some good ideas and precautions? 
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
If you had HgO it might work and distill over mercury. I don't think you could build a flask that would even melt MgO.
A definite hell no on getting magnesium like this.
Move to beginnings.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
MgO is used as a refractory, having a melting point of about 2800 C and a boiling point around 3600 C. That's two zeros at the end of thsoe numbers.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Once you have magnesium in one of its compounds, it is VERY hard to obtain the metal again. The processes, used in industry are not of the type, which
are easily done at home (requiring the handling of very hot molten substances). Forget about making Mg from your MgO.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
The industrial process to make magnesium metal requires you to convert the MgO (or other basic salts of Mg) to MgCl2 by action of HCl on the it.
Then the salt is melted down and an electric current is passed through. This reduces the Mg2+ to Mg and oxidizes Cl- to Cl2 (dangerous). It's going
to cost you more money to convert your MgO to Mg than just simply buying it.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
And for that matter, you'll only get MgCl2 if your MgO is reactive. Dead-burnt, refractory grade MgO, like the other refractory oxides ZrO2, Al2O3,
Cr2O3, etc., is very unreactive when recrystallized.
An alternate industrial process takes MgO and charcoal, heats them in a vacuum arc furnace and rapidly condenses the vapor. The reaction MgO + C
<--> CO + Mg(g) proceeds to the left at low temperatures, due to the high melting points of both reactants. At high temperature, some of the
righthand products are inevitably created as the mixture evaporates. Therefore, vacuum also helps. The gasses must be quenched so the magnesium can
be condensed or, more likely, sublimated before it recombines. The product is called a crown, which makes sense if you can imagine what such a
process might create. (In archaic terms it would be called "regulus of magnesium", but I suppose they didn't have vacuum arc furnaces to create and
name this stuff.)
If you are dead-set on the process, you'll probably want electrolysis on an eutectic mixture of NaCl, KCl and MgCl2. The cathode can be whatever,
while the anode should be something like graphite. I have heard molten MgCl2 is extraordinarily corrosive and requires very special materials, such
as inconel, to handle the stuff. Note also that, like sodium, magnesium floats in its molten salt, so you'll need a Downs cell to prevent corrosion.
Tim
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
Can it obtain Mg for calcination from MgCl2? I have read that it decomposes Mg + Cl2 at 300ºC.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Filemon
Can it obtain Mg for calcination from MgCl2? I have read that it decomposes Mg + Cl2 at 300ºC. |
Take whatever you read that in and throw it away
MgCl2 MP 708 C, BP 1412 C
http://www.freepatentsonline.com/3980536.html
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
It says here:
http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~DsX8r...
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
This is what your site says:
Melting Point:
712 deg C
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
But it also says:
Other Chemical/Physical Properties:
Slow heating releases chlorine @ 300 deg C; attacks fused silica when melted; easily forms alcoholates and etherates; deliquencent crystals; at 100
deg C loses 2 H2O (17.7%); at 110 deg C begins to lose some HCl; by strong ignition is converted into oxychloride.
[O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co.,
Inc., 2001., p. 1016]**PEER REVIEWED**
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
So? Lots of hydrated chlorides decompose. [Mg.6H2O]Cl2 gives off some water easily (as it says, at 100C), but for much of the remainder, the Mg atom
pulls too hard on the oxygen (I might have to term such atoms oxygenophiles  ),
causing the water "molecules" (they aren't really lone molecules of H2O anymore since parts of them are bound to other things) to dissociate and go
off with the Cl, which is more volatile and therefore a preferred product. HCl gas is the result. ),
causing the water "molecules" (they aren't really lone molecules of H2O anymore since parts of them are bound to other things) to dissociate and go
off with the Cl, which is more volatile and therefore a preferred product. HCl gas is the result.
I've heard from someone who was working to build a commercial Mg Downs cell that molten MgCl2 is extremely corrosive to almost anything. He had to
use corrosion-resistant superalloys to hold the stuff.
Tim
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
Then it happens hydrolysis as AlCl3·6H2O. MnO would take place. Certain? It seemed strange that it decompose so easily.
[Edited on 10-8-2007 by Filemon]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
The upper alkaline metals, namely Be and Mg, are worse bases than the lower ones, Ca, Sr and Ba. Mg may be adjecent to Na, but it's also adjecent to
Al, which we all know hydrolyses.
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The MgCl2 is stated to decompose slowly when heated, and I've found a similar reference. If may be hydrolysis from mosture in the air, or it may be a
reaction with oxygen, or both.
Oxychlorides are the products. Rule of thumb is that any metal that reacts with non-oxidising acids is unlike to have the metal form from halides, or
salts with oxygen containing anions, when heated. Nitrides and hydrides may form the metal upon heating, even for reactive metals.
|
|
|
16MillionEyes
Hazard to Others
  
Posts: 153
Registered: 11-3-2007
Location: 16 Million Eyes, US
Member Is Offline
Mood: No Mood
|
|
Displace by adding lithium metal. Obviously not a cheap nor very effective way of getting it. XD
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
OK, the reaction of Mg° --> to MgO is sufficiently exothermic that, once started, can abstract oxygen from dry ice, directly. The heat of reaction
(in excess of 2000°C) is something like -800kJ/mol, which is enormous. This is the barrier preventing you from going in reverse! This much energy,
must somehow be put back into the system (be it electrolytic, energy put into making Li metal, whatever) in order to make Mg°.
Neither trivial, nor cheap. It is much cheaper to hit the scrap-yard and find some old Mg lawnmower decks. Super cheap and large quantities.
I would be very impressed with anyone who could do this, at any scale, with an amateur set-up.
Best of luck and be careful,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
16MillionEyes
Hazard to Others
  
Posts: 153
Registered: 11-3-2007
Location: 16 Million Eyes, US
Member Is Offline
Mood: No Mood
|
|
That's one of the thing that bother me sometimes. How do you know which metal is which. You can easily go to a hardware store and find tons of metals
lying around but there's no easy way to say "this be Iron, that be zing etc". Any qualitative shortcuts to recognize Mg from other metals?
[Edited on 11-8-2007 by __________]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
The Mg decks are *very much* lighter. You can also carry a pocket knife (if you can cut off a sliver it would be Mg (Zn is not, that I know of, used
for mower decks). ALternatively, a small dropper-bottle of HCl (1:1, or less) is indicative; scratch off a small amount of paint or passivating layer,
to yield bare, shniy metal then apply a droplet. If you get immediate fizzing, you probably have Mg. Alternatively, an Mg test with Calmagite
indicator will also work.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
16MillionEyes
Hazard to Others
  
Posts: 153
Registered: 11-3-2007
Location: 16 Million Eyes, US
Member Is Offline
Mood: No Mood
|
|
Those are nice ways to test for Mg when doing it in a scrapyard of some sort but certainly not in a hardware store. Just imagine yourself getting
caught with a solution of HCl and a knife in a hardware store. Oh boy, you'll be in for some fun. Haha.
Unfortunately to avoid that type of situation you would have to buy a small piece of the metal in the hardware store and then by trial and error find
the metal. This is highly expensive in the long run though. Also, if you get a type of alloy by accident then there's much more trouble in telling
what is what.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Maybe you could ask someone at the hardware store? Or an industrial supplier. People do fabricate things from Mg (relatively easy to mill, strong,
lightweight, etc.).
The alloys can be worked out by dissolving in acid, then performing known qualitative tests (o-phenanthroline for Fe, dimethylglyoxime for Ni, etc.).
Tedious, but possible...unless you have XRF or EDAX, then not so tedious. (as
if!). not so tedious. (as
if!).
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Of the most common alloys:
Iron: heavy, magnetic. Austenitic stainless (304, 316, etc.) is mostly nonmagnetic, to slightly magnetic when worked (bent corners, drawn sides,
etc.). Hardened stainless (400 series) is magnetic. Chemical tests: myriad. Many alloys and superalloys may appear to be iron-based (nickel and
cobalt are also ferromagnetic, while many alloys of all three are not), but grouping them here is sufficient to do further testing on the stuff later.
Most alloys produce distinctive sparks, orange to yellow to white, when touched with a high speed abrasive grinder. Many alloys are refractory, and
many will oxidize when heated.
Aluminum: light, nonmagnetic, excellent conductor of heat and electricity. (A strong magnet drawn across the surface of the metal resists movement
due to eddy currents. This also applies to copper, silver and gold. Remember alloys in general are more resistive than pure metals.) Reacts with
HCl and NaOH with effervescence, but does not react with vinegar. Moderate melting point.
Zinc: heavy (as heavy as steel), moderate conductor. Low melting point and relatively low boiling point. Burns with distinctive blue/green flame
(not cyan; the color has two spectral lines).
Lead: very heavy, bad conductor of heat and electricity, low melting point. Not very reactive. Pb(2+) insoluble with Cl-, SO4(2-), etc.
Tin: heavy, low melting point. Often alloyed with lead. Sn(2+) ions are oxidizable to amphoteric Sn(4+).
Magnesium: light (lighter than aluminum), reacts vigorously with HCl, bubbles with vinegar (as distinguished from Al). Flakes and powder burn with a
bright white light; alloys sometimes give an orange glow as well. Moderate melting point (about the same as aluminum's) and relatively low boiling
point (somewhat higher than zinc's).
Titanium: moderate density (less than twice as heavy as aluminum, but half that of copper), corrosion resistant, refractory, bad conductor of heat and
electricity. Grinder gives bright white sparks. Reacts slowly with strong HCl or H2SO4 to give a purple solution of Ti(3+), which turns clear in
air, oxidizing to TiO(2+). Several alloys are common.
A lot can also be guessed based on the use. Zinc and zinc-aluminum alloys are common for die castings. Lead and tin are used in solder. Aluminum
and steel are used in common structural items. Special steel alloys and titanium are used in robust or special structural items.
Tim
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
| Quote: |
Note also that, like sodium, magnesium floats in its molten salt, so you'll need a Downs cell to prevent corrosion. |
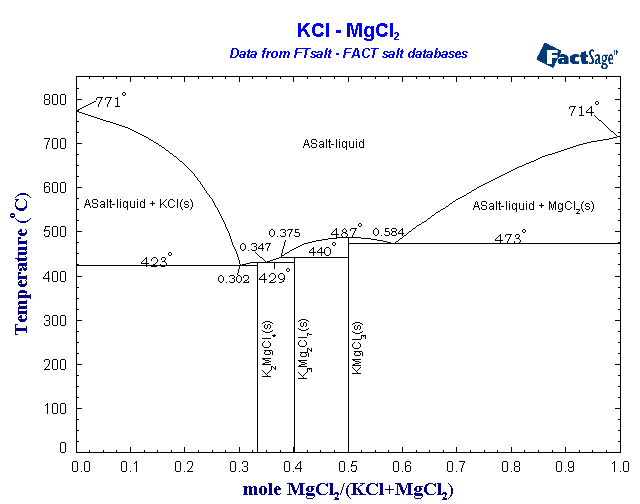
A eutectic mixture of MgCl2 and KCl would allow the cell to work at only about 450°C. I would not be surprised if a steel crucible could hold this
with minimal corrosion. More importantly, this means that the magnesium should collect as a solid on the cathode. Solid pieces may float to the top,
and the metal may need melted afterward to separate it from any infused salt, but the solid magnesium at this temperature should not need much
protection from atmospheric oxygen - perhaps just an argon layer in the open top of the cell, if anything. The chlorine generated by the cell is more
of a problem. It will need to be funneled out and reacted with something, in a reaction that produces little to no back-pressure. Aqueous FeCl2 or
aqueous particulate Fe --> FeCl3 is probably the easiest way to absorb chlorine in a manner that leads to a somewhat useful product. This process
is involved enough already that you will be losing out if you just bubble it through sodium bicarbonate or something stupid like that. You're
producing pure anhydrous chlorine so why use it to convert more expensive bases into worthless NaCl? The hot chlorine could even better be used in an
adjacent vessel to form volatile chlorides with carbon/chloride reduction, such as TiCl4, BCl3, etc. CCl4, S2Cl2, NOCl, and a host of other useful
reagents could also be prepared by taking advantage of the hot dry chlorine. Another possibility, probably the most useful if it would work, is
making more anhydrous MgCl2 with the chlorine according to the reaction MgO + Cl2 + C --> MgCl2 + CO.
Below is a phase diagram of the MgCl2/KCl system. You'd simply run the cell until you see crystals of KCl start to form, then add more MgCl2 until no
more will dissolve, keeping the levels within the liquidus area this way for the duration of the run.
[Edited on 22-10-2008 by kilowatt]
The mind cannot decide the truth; it can only find the truth.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
As as alternative to conversion of MgO to MgCl2, which may not be possible with solid fused MgO, and electrolysis of the molten MgCl2 or the eutectic
mixture with KCl or NaCl, I wonder if an electrolytic process analogous to the smelting of Al directly from refined Al2O3 could be used, involving
its dissolution in molten Na3AlF6 (or a stoichiometric mixture of NaF and AlF3, which is continuously re-created in the process). Such a process would
involve dissolution of MgO in a mixture of molten MgF2 and NaF or KF. Has that ever been tried for Mg directly from MgO?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I heard that molten MgCl2 is extremely corrosive, and cells must be constructed of monel or inconel stuff just to keep them from dissolving.
The thing about magnesium is, the chloride is a textbook chloride, not like that unbehaved aluminum compound, so there is no need for fluorides and
oxides and spent graphite.
I have no idea what the solubility of MgO in MgF2 or other fluoride mixtures would be. Probably small as MgO has such a large delta H.
Tim
|
|
|
| Pages:
1
2 |