| Pages:
1
2
3 |
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Production of Cu (I) Cl with household chemicals. Problems.
I'm working on producing CuCl I'm interested in doing it from easily available starter chemicals found at home, or hardware stores;
I attempted following a recepie on another thread: https://www.sciencemadness.org/whisper/viewthread.php?tid=62...
However, using table salt produced Cu2(OH)3Cl which is green, and not the expected white precipitate of CuCl.
So, I'm looking at ways of doing the same process using other commonly available chemicals found at hardware stores and grocery stores. My first
attempt will be described below. It failed, and I am wondering what I did wrong.
I am presently using CuSO4 root killer (99%) , reducing sugar (I used Kitchen alchemy brand glucose powder, later I may try Karo Corn syrup/ glucose
syrups), muriatic acid, and washing soda Na2CO3
According to Wikipedia, the conversion can be achieved with a reducing sugar "like" ascorbic acid; https://en.wikipedia.org/wiki/Copper(I)_chloride
NOTE: I have both ascorbic acid and sodium metabisulfite as backups to do tests with but am avoiding using them as less common chemicals than Karo
Syrup/glucose.
I decided to attempt to purify the chemicals somewhat by first converting copper sulfate into basic copper carbonate and then washing the result with
Reverse Osmosis water. From a bit over 2.5 grams of CuSO4.5H20, I got 1.010 grams of basic copper carbonate after drying at 45C overnight.
That works out to 0.00817 moles of basic copper carbonate:
Cu2(OH)2CO3 --> HO-Cu-O-C-O-Cu-OH
Where the schematic carbon has one additional oxygen with double bond, not shown.
My plan was to try and reduce both -OH radicals on the end of the molecule, and then use the hydrochloric acid to attack the carbonate and replace
both it's oxidation bonds.
Since water / OH has a pka of 14.995, Carbonic acid a pka1,2=6.35, 10.33, and HCl a pka of -6 ; that determines which acid is most easily attacked,
vs. the hardest.
So, I assume a reducing sugar ought to attack the OH from water before attacking the carbonic acid.
So, I added 2*0.00817 moles =2.944(1) grams of glucose to the basic copper carbonate, and then I added 30ml of RO water. No bubbling occurred, so the
carbonic acid still remained. A very small amount of brown dotting appeared on the glass, which I assume is an impurity in the glucose that reacted
with the copper carbonate. It was a very minor amount obviously being less than 1% of the volume of powder in the flask.
In order to get CuCl (1:1), I also need 2*0.00817 moles of hydrogen chloride which I got from ace hardware as muriatic acid, 31.45% solution.
This is how I tried to get approximately the correct number of moles:
I poured 50ml into a precision volumetric flask; I believe it's calibrated for 20C. The room was 21C, so there shouldn't be an error over -50mg due to
temperature. I weighed it and after removing flask tare, I got 57.376g of HCl solution in 50ml volume = 1.14752 g/cc (@ 21C). ( it might be a bit
more at 20C, like 1.15 g/cc )
So, I think that means I have 1150 g/L * 31.45% = 361.7 g Hcl/L
So, that's 361.7g/L / 36.46 g/mol ~= 9.92 mol/L (I rounded to 9.9 molar ).
Therefore, I weighed out 2*0.00817mol/9.9 mol/L = 0.00165L of liquid, by it's weight of 1.893 grams. All mass measurements are +-1 milligram as I
have a digital scale.
Then I added the HCL dilluted to 20ml with RO water, drop by drop to the flask with magnetic stirring and the plate held at 50C. The color of the
stirred mixture started out green, and lightened slowly to the halfway point of the acid being dripped in. However, after the halfway point, the
solution started becoming clear and blueish. There was absolutely no precipitate, except that the tiny bit of brown stuff that I mentioned earlier
floated to the top.
So I filtered it out with a coffee filter, and returned the solution to the flask. The solution is perfectly clear with a light blue color, and there
is absolutely no precipitate. There is not enough HCl to make copper II chloride out of all the copper, unless I made a mistake, and all the copper I
chloride-hydroxides are supposed to be insoluble. It's been stirring for hours at 50C and there is no precipitate.
What did I do wrong? I would expect that due to leakage of HCL into air, that there would be less HCL in the jug than the percentage when it was new.
Yet, the blue color seems to indicate that I have added more HCl than needed. It also suggests a reducing sugar "like" ascorbic acid (glucose is a
reducing sugar) isn't good enough.
  
[Edited on 29-11-2017 by semiconductive]
Note: I just weighed 50ml of RO water, and came up with 49.673g. That mass is under what is expected for 71F room, grade A #5560 TC20 flask. There's
tiny air bubbles forming in the RO, so maybe it's that ... but its an error of about 200mg ... which is huge. I'm going to heat the water, and let
it cool and then re-measure.
[Edited on 29-11-2017 by semiconductive]
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Copper (II) sulphate + NaCl => Cu(II)Cl2
Cu(II) Cl2 + sodium metabisulphite => Cu(I)Cl ppt
Decant and rinse and decant again.
Total bucket chemistry in a one pot synth.
Edit:
I know you wanted to use reducing sugars but with such strong ions like Cl- sugar isn't going to be able to pull that off, tollens reaction uses a
fairly weak cation like silver and ammonia to basically balance the force of the nitrate then the sugar/aldehyde can get in there and do it's thing.
[Edited on 29-11-2017 by Chemetix]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Take your green hydroxide/chloride and dissolve it in hydrochloric acid (muriatic acid), add some electrical-grade copper wire, and seal it. The
solution will be yellow if all of the copper is in the +2 state, and brown-black if it's a mixture of +1 and +2. Shake it with the wire until the
solution goes colourless- this may take overnight.
Dilute the solution by rapid addition of boiled water, and white CuCl should precipitate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
@chemetix,
I've seen several videos of sodium metabisulfite being used to make Cu(I)cl ppt. I'm Not wanting to do that until I've exhausted the reducing sugars
approach.
I'm wanting to understand how the chemistry works / should work ... and why my test didn't.
As to your post:
I am curious about what drives the metabisulfite reaction at all, or how sodium gets stripped from chlorine given the pka of HCl is -7, but that of
SO4 is -3 or +2.
In all the successful metabisulfite experiments I've seen ,they first neutralize metabisulfite with sodium. Na2CO3, or NaOH.
eg:https://www.youtube.com/watch?v=6H-c_tB5ToM&t=194s
You don't neutralize it...
In your suggestion, the sodium is stripped by the sulfate to make sodium sulfate and copper II chloride. So, there is some sodium to neutralize the
bisulfite, but only if the Sulfate ion gives up the sodium. Could you explain why the experiment would still work with the PH of the solution going
very acidic? I mean, will copper I chloride remain a precipitate with free sulfuric acid around? or will some ppt re-dissolve into solution?
I'm thinking the idea's strange that when NaCl is exposed to sulfuric acid that the sulfate steals the sodium and liberates HCl given the pka's of the
acids; so that free HCl can then attack the copper. I'm sure that sodium is more electro-active than copper, so I would expect chloride to want the
sodium more than the copper. In most metastasis reactions the strongest base ions pair with the strongest acid ions ... leaving the weaker acid and
base ions to neutralize each other.
What drives the reaction that you are proposing?
[Edited on 29-11-2017 by semiconductive]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by semiconductive  |
But I am curious about what drives the metabisulfite reaction at all, or how sodium gets stripped from chlorine given the pka of HCl is -7, but that
of SO4 is -3 or +2. |
The sodium isn't "stripped" from anything- the sodium is an ion, and is only hanging around the chloride or sulphate because they've got opposite
charges. Once it's dissolved in aqueous solution, the sodium ion is solvated by water, and couldn't care less about the anions.
| Quote: | | In your suggestion, the sodium is stripped by the sulfate to make sodium sulfate and copper II chloride. So, there is some sodium to neutralize the
bisulfite, but only if the Sulfate ion gives up the sodium. Could you explain why the experiment would still work with the PH of the solution going
very acidic? I mean, will copper I chloride remain a precipitate with free sulfuric acid around? or will some ppt re-dissolve into solution?
|
No- the sulphite ion can reduce the copper(II) to copper(I) only when there are chloride ions around to stabilize copper(I) as the insoluble chloride.
The sodium ions aren't involved in the reaction at all, and are only there because you can't add chloride without some cation along with it. The pH
for this reaction scarcely matters because chloride is a very weak base, and H+ ions will not interfere with the precipitation of the CuCl.
[Edited on 29-11-2017 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by DraconicAcid  | Take your green hydroxide/chloride and dissolve it in hydrochloric acid (muriatic acid), add some electrical-grade copper wire, and seal it. The
solution will be yellow if all of the copper is in the +2 state, and brown-black if it's a mixture of +1 and +2. Shake it with the wire until the
solution goes colourless- this may take overnight.
Dilute the solution by rapid addition of boiled water, and white CuCl should precipitate. |
OK. That's similar to the idea I was told to do on the other thread.
The only difference is that they told me to boil them all together, and you're telling me to first let them sit until it's colorless and then add
boiling water.
In the experiment I tried, I put copper wire, salt, and copper sulfate in a jar. There was a stoichometric mix of salt and copper sulfate. The color
of the solution went green immediately. The cap temperature was set at 40 degrees, for several hours. During that time, the copper wire was coated
with a precipitate that could have been white or light green and no other change to the greenish solution happened. (It's hard to tell copper wire
color inside green solution). At the end of several hours, I began raising the temperature to see if the copper wire would clear up ... and it did
as I got above 70C. By 80C, the wire was clean. As I approached boiling, the green precipitate began forming (described in other thread). https://www.sciencemadness.org/whisper/viewthread.php?tid=62...

So, my solution was always greenish. Neither yellow nor brown black were ever seen.
Is this due to a contaminant in the morton salt, KI, or the anti-caking agent?
1st: Given your process, will it work with stoichiometric 2 NaCl + CuSO4 --> Na2SO4 + CuCl2 , or does there have to be excess HCl for it to work,
and why?
2nd: What is the maximum temperature that the solution ought to be kept at overnight to dissolve the wire?
[Edited on 29-11-2017 by semiconductive]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
If you have time the simplest method is to place bare copper wire or bits of copper in a jar and fill it up with dilute ammonium chloride solution and
seal tightly. Cuprous chloride crystals grow slowly as magnificent complex modified cubic crystals to 8 mm or more, though it take years to get large
ones. I discovered this process by accident but it yields very nice crystals which oxidize more slow on exposure than the powdered form. When I opened
my jar after many years I found that there was no smell of ammonia which I find rather curious.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That's because you had a lower concentration of chloride ion.
| Quote: | | 1st: Given your process, will it work with stoichiometric 2 NaCl + CuSO4 --> Na2SO4 + CuCl2 , or does there have to be excess HCl for it to work,
and why? |
Higher concentrations of chloride help keep copper(I) in solution so that it doesn't coat the wire. Wire won't react if it's coated.
| Quote: | | 2nd: What is the maximum temperature that the solution ought to be kept at overnight to dissolve the wire? |
I don't think it matters, as long as HCl isn't evaporating.
[Edited on 29-11-2017 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Chemetix  | Copper (II) sulphate + NaCl => Cu(II)Cl2
Cu(II) Cl2 + sodium metabisulphite => Cu(I)Cl ppt
Decant and rinse and decant again.
Total bucket chemistry in a one pot synth.
|
Beautiful.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by Boffis  | | If you have time the simplest method is to place bare copper wire or bits of copper in a jar and fill it up with dilute ammonium chloride solution and
seal tightly. Cuprous chloride crystals grow slowly ... When I opened my jar after many years I found that there was no smell of ammonia which I find
rather curious. |
I'm buying 2 dram vials with phenolic screw on caps so they can be heated up to 150 to 200C under pressure. It's no biggie to leave one of those with
the solution in it on an unheated rack and see what happens. I'm more concerned with safely producing ammonium chloride without making ammonia and
chlorine gas... probably something to do outside!
I don't think it's all that strange you didn't smell ammonia. I made a copper oxide powder (black), and placed it in aqeous ammonia (15%) with oil
over it to prevent air from getting in. The ammonia did not dissolve the copper oxide, as far as I could tell, even after a week of sitting. But,
after removing the oil the same bottle exposed to air began forming an indigo colored solution within minutes especially if I blew across the water.
What is going on is that CO2 from the air attacks the copper oxide and the aqeuous ammonia; producing ammonium carbonate and possibly copper
carbonate. The ammonia odor becomes very faint over time, as complexed ammonia with copper is more stable than ammonia carbonate by itself.
I would think that the ammonia chloride in your solution would also make a complex with copper chloride, as it forms. So, there ought to be both
copper chloride (hydroxide?) held in solution by ammonia, and your precipitated crystals.
Did you weigh the remaining solution to get any quantitative idea of how much dissolved material was still in solution after the crystals
precipitated?
I'm pretty disgusted with my pyrex volumetric flasks. Before I do your experiment, I am going to flame polish the bottom and then see if I can deform
the concave portion of the bottom to correct the -0.200ml error in volume that I measured. The flask says +-0.05ml, but I'm getting a lot bigger error
than marked. If you have any suggestions for how to accurately deform glass ... I'll be thinking about ways to do that tonight.
[Edited on 29-11-2017 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by semiconductive  |
But I am curious about what drives the metabisulfite reaction at all, or how sodium gets stripped from chlorine given the pka of HCl is -7, but that
of SO4 is -3 or +2. |
The sodium isn't "stripped" from anything- the sodium is an ion, and is only hanging around the chloride or sulphate because they've got opposite
charges. Once it's dissolved in aqueous solution, the sodium ion is solvated by water, and couldn't care less about the anions.
|
I'm after an understanding of the principles involved, not just making chemicals. I'm wanting to know why my experiment didn't work, as well as why
yours will.
Charges don't have brains, or "care"; I get that. However, I've been taught that semiconductor physics is analogous to aqueous chemistry. I
originally took chemistry as an EE. The idea my chem teacher gave is a little more complicated than what you seem to be saying.
In semiconductor physics, free wandering "ions" in the crystal are attracted to the opposite charge just like ions in water. There are "bound"
states, where an ion (fermion) hangs around the oppositely charged ion but never wandering more than a few angstroms away; eg: in reality the "bound"
ion is actually "dissolved" in the semiconductor crystal in a molecular orbital. It typically only wanders around one to two atoms away from the ion
that it is actually attracted to. When the temperature of the semiconductor is raised high enough, the free ions can get separated for a short period
of time and "hop" from one impurity ion to another; but otherwise they stay fairly near their oppositely charged ions.
I would expect the same is true in solutions, ions stay near each other and only switch when charges (electronegativity) is greater or thermal
agitation gives them enough energy to escape the ion they are attracted to.
Note: In the youtube video I gave, I think he said sodium sulfite, and perhaps thats different than sodium metabisulfite. I was thinking that they
were the same and metabisulfite has a spare hydrogen atom to create free sulphuric acid. I think that's probably most of my misunderstanding of what
you said.
If metabisulfite has two sodiums, then my original thought was misguided; there wouldn't be free hydrogen to from sulfuric acid with in the first
place. The stochiometry is good for pure sodium sulfate which is pretty close to neutral. Sodium meta-bisulfate apparently has a pH of around 3.5 to
5. So, it's apparently an acidic salt.
| Quote: |
| Quote: | | In your suggestion, the sodium is stripped by the sulfate to make sodium sulfate and copper II chloride. ... I mean, will copper I chloride remain a
precipitate with free sulfuric acid around? or will some ppt re-dissolve into solution? |
No- the sulphite ion can reduce the copper(II) to copper(I) only when there are chloride ions around to stabilize copper(I) as the insoluble chloride.
The sodium ions aren't involved in the reaction at all, and are only there because you can't add chloride without some cation along with it. The pH
for this reaction scarcely matters because chloride is a very weak base, and H+ ions will not interfere with the precipitation of the CuCl.
|
OK. you've said something here very detailed.
The conjugate base for chloride is basically non-extant, for the pka is like < -3; so I follow you about the weak base point.
But if "Chloride" ions stabilize the copper (I), then let me ask a different question;
qualitatively, if I had pure white powder CuCl in the bottom of a flask, no oxygen or air allowed in, and I added HCl to the water; How do I know that
the HCl won't be able to oxidize the copper to the +2 state, and release hydrogen gas? What's the theoretical grounds for it reacting or not
reacting?
Likewise, what's the theoretical reason that we know that adding bisulfite as free acid, something like: [Na+] [H+] [S2O5]-- won't release the
hydrogen to oxidize the copper to the +2 state?
As an abstraction of both the previous ideas, I'd ask the same essential question like this:
If I add excess HCl acid to the solution containing metabilufite, why wouldn't the free acid be able to release hydrogen and attack the CuCl?
With that being said, I know that the equation as described by you will work; I'm just trying to understand if it would work if the pH of the
solution was reduced by a free acid's [H+] ions.
[Edited on 30-11-2017 by semiconductive]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by semiconductive  | | I'm more concerned with safely producing ammonium chloride without making ammonia and chlorine gas... probably something to do outside!
|
No, just mix dilute hydrochloric acid with dilute aqueous ammonia. NEVER mix chlorine in any form with ammonia- nitrogen trichloride is touchy stuff
(Pierre Dulong, who first made it, lost two fingers and an eye to it).
| Quote: | | I don't think it's all that strange you didn't smell ammonia. I made a copper oxide powder (black), and placed it in aqeous ammonia (15%) with oil
over it to prevent air from getting in. The ammonia did not dissolve the copper oxide, as far as I could tell, even after a week of sitting. But,
after removing the oil the same bottle exposed to air began forming an indigo colored solution within minutes especially if I blew across the water.
What is going on is that CO2 from the air attacks the copper oxide and the aqeuous ammonia; producing ammonium carbonate and possibly copper
carbonate. The ammonia odor becomes very faint over time, as complexed ammonia with copper is more stable than ammonia carbonate by itself.
|
Carbon dioxide has nothing to do with this. Copper oxides will dissolve slowly in ammonia to give either the dark blue tetramminecopper(II) ion or
the colourless diamminecopper(I) ion (along with hydroxide ion). In your case, it appears there was enough unreacted copper or copper(I) oxide that
the latter was formed. Exposing it to air caused oxidation to the copper(II) ion, which is very strongly coloured.
| Quote: | | I would think that the ammonia chloride in your solution would also make a complex with copper chloride, as it forms. So, there ought to be both
copper chloride (hydroxide?) held in solution by ammonia, and your precipitated crystals. |
Ammonium chloride (not ammonia chloride) can't form a complex- it takes free ammonia molecule to coordinate to a metal (it needs a free lone pair of
electrons to act as a ligand).
| Quote: | | I'm pretty disgusted with my pyrex volumetric flasks. Before I do your experiment, I am going to flame polish the bottom and then see if I can deform
the concave portion of the bottom to correct the -0.200ml error in volume that I measured. The flask says +-0.05ml, but I'm getting a lot bigger error
than marked. If you have any suggestions for how to accurately deform glass ... I'll be thinking about ways to do that tonight.
|
I suspect that will only make them worse.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by semiconductive  |
I'm after an understanding of the principles involved, not just making chemicals. I'm wanting to know why my experiment didn't work, as well as why
yours will.
Charges don't have brains, or "care"; I get that. However, I've been taught that semiconductor physics is analogous to aqueous chemistry. I
originally took chemistry as an EE. The idea my chem teacher gave is a little more complicated than what you seem to be saying.
In semiconductor physics, free wandering "ions" in the crystal are attracted to the opposite charge just like ions in water. There are "bound"
states, where an ion (fermion) hangs around the oppositely charged ion but never wandering more than a few angstroms away; eg: in reality the "bound"
ion is actually "dissolved" in the semiconductor crystal in a molecular orbital. It typically only wanders around one to two atoms away from the ion
that it is actually attracted to. When the temperature of the semiconductor is raised high enough, the free ions can get separated for a short period
of time and "hop" from one impurity ion to another; but otherwise they stay fairly near their oppositely charged ions.
I would expect the same is true in solutions, ions stay near each other and only switch when charges (electronegativity) is greater or thermal
agitation gives them enough energy to escape the ion they are attracted to. |
No. When an ionic compound like sodium chloride or sodium sulphate are dissolved in water, the ions become hydrated. The sodium ions hang around
with the water molecules, and have very little to do with the chloride ions unless the concentration is very high. I wasn't trying to point out that
sodium ions don't have brains- I always anthropomorphize ions and atoms because I'm usually explaining these things to kids. The sulphate ion in a
solution of sodium sulphate will act identically to a sulphate ion in a solution of ammonium sulphate, potassium sulphate, cesium sulphate, or even
aluminum sulphate- the ions wander far enough away that the sulphate has almost no interaction with the cation.
| Quote: | | Note: In the youtube video I gave, I think he said sodium sulfite, and perhaps thats different than sodium metabisulfite. I was thinking that they
were the same and metabisulfite has a spare hydrogen atom to create free sulphuric acid. I think that's probably most of my misunderstanding of what
you said. |
They are different, but related. Sodium metabisulphite (Na2S2O5) will react with water to give aqueous sodium hydrogen sulphite (NaHSO3)
| Quote: | | Sodium meta-bisulfate apparently has a pH of around 3.5 to 5. So, it's apparently an acidic salt. |
Correct
| Quote: | But if "Chloride" ions stabilize the copper (I), then let me ask a different question;
qualitatively, if I had pure white powder CuCl in the bottom of a flask, no oxygen or air allowed in, and I added HCl to the water; How do I know that
the HCl won't be able to oxidize the copper to the +2 state, and release hydrogen gas? What's the theoretical grounds for it reacting or not
reacting? |
If you look up the reduction potentials of copper, you'll find that they are positive for both copper and copper(I), which means that H+ will not
oxidize either of them.
| Quote: | | Likewise, what's the theoretical reason that we know that adding bisulfite as free acid, something like: [Na+] [H+] [S2O5]-- won't release the
hydrogen to oxidize the copper to the +2 state? |
Because H+ isn't a strong enough oxidizing agent to turn either copper or copper(I) into copper(II).
| Quote: | | If I add excess HCl acid to the solution containing metabilufite, why wouldn't the free acid be able to release hydrogen and attack the CuCl?
|
Because the acid (H+) won't be able to react with the chloride (it's too weak of a base) or the copper(I) (it's too weak of a reducing agent).
| Quote: | | With that being said, I know that the equation as described by you will work; I'm just trying to understand if it would work if the pH of the
solution was reduced by a free acid's [H+] ions. |
H+ ions shouldn't make much difference, as long as it's dilute enough that it's still an aqueous solution (and not 90% sulphuric acid).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by semiconductive  | | I'm more concerned with safely producing ammonium chloride without making ammonia and chlorine gas... probably something to do outside!
|
No, just mix dilute hydrochloric acid with dilute aqueous ammonia. NEVER mix chlorine in any form with ammonia- nitrogen trichloride is touchy stuff
(Pierre Dulong, who first made it, lost two fingers and an eye to it).
|
Thanks for all the descriptive input. As I learn more, I'll definitely work my way through all the things you have said here. I was never planning
to mix chlorine with ammonia, I'm just aware than mixing aqueous solutions of bleach and ammonia produce both ammonia gas and chlorine gasses, that
have hurt lots of house-keepers cleaning toilets. With my limited experience, I wasnt' sure if HCl(aq.) and ammonia (aq.), could also react to release
chlorine or ammonia gasses. Case in point, Pierre didn't know what he was doing either ... so better safe than sorry. 
| Quote: |
| Quote: | | I'm pretty disgusted with my pyrex volumetric flasks. Before I do your experiment, I am going to flame polish the bottom and then see if I can deform
the concave portion of the bottom to correct the -0.200ml error in volume that I measured. The flask says +-0.05ml, but I'm getting a lot bigger error
than marked. If you have any suggestions for how to accurately deform glass ... I'll be thinking about ways to do that tonight.
|
I suspect that will only make them worse. |
It's only one flask which seems to be so far "off".
That's why I'm remeasuring with distilled water, and R.O. water after heating to degass them to make sure I didn't make some kind of mistake. If it's
not micro gas bubbles which have caused the problem, It could only be a major factory defect, or extreme abuse by a previous owner, that would make
the flask be off by this much.
I checked the calibration on the mass scale, and it's fine. I'm only 100 feet above sea level, so I assume that I'm close enough to standard pressure
to not affect 50cc's of waters volume by 0.2cc.
If I haven't made a mistake, then my only choices are to re-mark the neck with a second precision scribe (hard to do), or soften the glass on the
bottom of the flask enough to make it less concave. Ordering another flask is always possible if I totally ruin it, but that takes a lot more time
and money. So, I'm willing to give fixing it a shot as I have nothing to loose.
[Edited on 30-11-2017 by semiconductive]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I think your best best would be to re-mark the volume with a very thin piece of electrical tape or duct tape. It's easy, close enough, and not going
to come off under normal washing conditions.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by semiconductive  | | I was never planning to mix chlorine with ammonia, I'm just aware than mixing aqueous solutions of bleach and ammonia produce both ammonia gas and
chlorine gasses, that have hurt lots of house-keepers cleaning toilets. With my limited experience, I wasnt' sure if HCl(aq.) and ammonia (aq.), could
also react to release chlorine or ammonia gasses. |
That's a myth. Acids mixed with bleach will produce chlorine gas; ammonia mixed with bleach will produce chloramines, which are also toxic.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
| Quote: | Quote: Originally posted by DraconicAcid  | Quote: Originally posted by semiconductive  |
| Quote: | But if "Chloride" ions stabilize the copper (I), then let me ask a different question;
qualitatively, if I had pure white powder CuCl in the bottom of a flask, no oxygen or air allowed in, and I added HCl to the water; How do I know that
the HCl won't be able to oxidize the copper to the +2 state, and release hydrogen gas? What's the theoretical grounds for it reacting or not
reacting? |
If you look up the reduction potentials of copper, you'll find that they are positive for both copper and copper(I), which means that H+ will not
oxidize either of them.
|
|
In the table of reduction potentials, copper solid metal to Cu++ is +0.34V, and Cu+ is +0.52V.
I also notice that more electric field / potential energy is required to cause a reduction of the single ionized copper, than that of the doubly
ionized copper atom.
That's odd ... intuitively, I'm used to working with atomic calculations in vacuum. removing the first electron generally takes less electric
field/energy than removing the second electron.
In the reduction potential chart, I notice that Ag+'s reduction is at 0.8V, and Ag++'s at 1.98; which follows the same trend as I am used to in
vacuum. That suggests to me, that water may have an "average" reference voltage that is analogous to a vacuum/ground reference at infinity for normal
physics and electronics calculation purposes.
I am guessing it's going to be be a potential > about +0.74 volts with respect to a hydrogen electrode as that's the minimum voltage required to
cause all ions on the reduction potentials charts (that I can see) to agree with the principle that first ionizations are always lower magnitude to
the reference voltage than the second ions.
In any event, copper is easier to oxidize to the +2 state, than it is to the +1 state with respect to a hydrogen electrode and that's
counter-intuitive; but I can accept it. Reference voltages are arbitrary.
However, that suggests copper will not be attacked at all by hydrochloric acid.
When I take copper, and put it in hydrochloric acid; it will generally clean off the oxide very quickly, if any exists. But even after the copper is
clean, hydrochloric acid will continue to attack copper slowly .. and faster as the temperature is raised.
The prediction of the reduction potential chart seems hard to believe/wrap my head around. I use HCl to etch copper circuit boards.
So, what you're telling me is something I'm going to have to test out.
I think I have to place the copper in HCl with non access to oxygen, air, to prevent oxidation (silicone oil or freon capping the liquid in an
air-tight way) and according to the chart that would suggest HCl could no longer attack the copper metal at all at room temperature (21C) at my
house.
This also brings up the problem of temperature that I've been wondering about.
In semiconductor physics, the law of mass action can be derived by taking the fermi level into account and the density of states of the secmiconductor
in "band" theory. I forget exactly how it was done, but the ions concentrations being simple products was an approximate result of the derivation;
but it was in fact dependent both on temperature and ion concentration (slightly.)
In Chemistry, I think the law of mass action is just accept a-priori / empirically ... but the chemical potentials are going to have to change
significantly with respect to temperature; eg: something like:
~ - k1 * RT ln ( k2 )
I know batteries change by millivolts/degree Celsius; which means the oxidation/reduction potentials must also vary with temperature. I note the
chart in my book says at "25C", so that's suggestive that the chart is increasingly invalid at other temperatures.
Just as a crude low-balled order of magnitude (guess) of 4millivolts/*C would mean that the Cu +0.34V potential could be totally overcome by a change
of 85 degrees Celsius. If the temperature change is in the right direction. Seeing that HCl etches copper faster as it gets hotter, I would expect
that heat could cause the lowering of the threshold that the chemical reaction takes place at.
[Edited on 30-11-2017 by semiconductive] |
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by semiconductive  |
In the table of reduction potentials, copper solid metal to Cu++ is +0.34V, and Cu+ is +0.52V.
I also notice that more electric field / potential energy is required to cause a reduction of the single ionized copper, than that of the doubly
ionized copper atom.
That's odd ... intuitively, I'm used to working with atomic calculations in vacuum. removing the first electron generally takes less electric
field/energy than removing the second electron. |
In vacuum, this is true, but in solution, the water more strongly coordinates the divalent cation and stabilizes it more. Copper(I) ions really don't
exist in aqueous solution, unless they are coordinated to a ligand that stabilizes them more than water does. Otherwise, 2 Cu(+) -> Cu(2+) +
Cu(s).
There are many metals which do not form stable +1 ions, and thus the ions with higher charges are easier to make. You will never find a reduction
potential for, say, zinc(I) or chromium(I)- these ions don't exist.
| Quote: | | That suggests to me, that water may have an "average" reference voltage that is analogous to a vacuum/ground reference at infinity for normal physics
and electronics calculation purposes. |
I do not know what you mean by this- water is not analogous to a vacuum in any way.
| Quote: | | I am guessing it's going to be be a potential > about +0.74 volts with respect to a hydrogen electrode as that's the minimum voltage required to
cause all ions on the reduction potentials charts (that I can see) to agree with the principle that first ionizations are always lower magnitude to
the reference voltage than the second ions. |
Aqueous ions won't agree with that principle, otherwise you would find aluminum(I) and aluminum(II) on your tables of reduction potentials.
| Quote: | However, that suggests copper will not be attacked at all by hydrochloric acid.
When I take copper, and put it in hydrochloric acid; it will generally clean off the oxide very quickly, if any exists. But even after the copper is
clean, hydrochloric acid will continue to attack copper slowly .. and faster as the temperature is raised. |
The hydrochloric acid will clean off the oxide very quickly- this is an acid-base reaction and is not affected by the redox potentials.
Once the copper is clean, you have copper ions and chloride ions in solution. In the presence of a high concentration of chloride ions, copper(I)
forms a stable complex ion called dichlorocuprate(I) [CuCl2]-. This will react with oxygen in solution or in the air to form copper(II) ions, which
then react with metallic copper in the presence of chloride ions to give more dichlorocuprate(I) ions....which then react with oxygen.
It is not the reaction Cu + 2 HCl -> H2 + CuCl2, which is thermodynamically unfavourable (as indicated by the redox table).
It is the overall reaction 2 Cu + 4 HCl + O2 -> 2 CuCl2 + 2 H2O, which is autocatalyzed by the presence of CuCl2.
This reaction will happen faster at higher temperatures because all reactions happen at higher temperatures, unless it's a process disfavoured by high
temperatures (like the freezing of water).
| Quote: | | In Chemistry, I think the law of mass action is just accept a-priori / empirically ... but the chemical potentials are going to have to change
significantly with respect to temperature; |
We can derive the law of mass action from thermodynamics.
| Quote: | | I know batteries change by millivolts/degree Celsius; which means the oxidation/reduction potentials must also vary with temperature. I note the
chart in my book says at "25C", so that's suggestive that the chart is increasingly invalid at other temperatures. |
That's true, they will change with temperature. If you have the reaction enthalpy, you can calculate the equilibrium constant at any other
temperature easily enough, and from there you can find the reaction potential.
| Quote: | | Just as a crude low-balled order of magnitude (guess) of 4millivolts/*C would mean that the Cu +0.34V potential could be totally overcome by a change
of 85 degrees Celsius. If the temperature change is in the right direction. Seeing that HCl etches copper faster as it gets hotter, I would expect
that heat could cause the lowering of the threshold that the chemical reaction takes place at. |
I'll do the calculation for you when I have time, but i doubt it will work that way. It's just a kinetic effect of reactions happening faster at
elevated temperatures.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by Chemetix  | Copper (II) sulphate + NaCl => Cu(II)Cl2
Edit:
I know you wanted to use reducing sugars but with such strong ions like Cl- sugar isn't going to be able to pull that off, tollens reaction uses a
fairly weak cation like silver and ammonia to basically balance the force of the nitrate then the sugar/aldehyde can get in there and do it's thing.
[Edited on 29-11-2017 by Chemetix] |
@Chemetix,
I Missed this comment from before.
How do I find out the strength of a reducing sugar, compared to Cl- , OH-, and HCO3- ?
More importantly, why would it need to remove Cl-, when the copper atoms are not oxidized with two chlorines in the first place, but only one?
I only added enough HCl to attack half the basic copper carbonate bonds;
Did I miscalculate the molar concentration of HCl? Would you check for me?
Basic copper carbonate is in the Cu+2 oxidation state. The +2 is caused by BOTH hydroxil anion, and a carbonate anion. I didn't supply enough HCl to
replace all the CO2 and OH. Therefore, one of the bonds must still be attached to the copper ... because the copper did all dissolve into solution
once the acid was added; I don't think I created a solution where chloride attacked only some of the basic copper carbonate, and left part of it
alone. Otherwise, why did all the copper carbonate go into solution?
I assumed either the hydroxide or the carbonate would be easier to displace, and the copper atom would stay in the +2 oxidation state because one of
the anions was HCl, but the OTHER anion would still be either hydroxide or carbonate.
My hope was that a reducing sugar would have an advantage in a situation where chloride was NOT the only anion attached to the copper, but a weaker
anion was present.
Besides, what you've told me is inconsistent or grossly over-simplified compared to what other people are saying.
The Wikipedia article I was following to make copper I chloride explicitly said that reducing sugars (eg: ones LIKE ascorbic acid / vitamin C ) was
able to reduce copper II chloride to copper I chloride. So, they are saying a reducing sugar can even overcome chloride. But you say it can't?
Wikipedia doesn't specify under what conditions it happens. (eg: Do you have to dry out the solution, or does it precipitate, etc. )
The article only said on December 14,2017
| Quote: |
https://en.wikipedia.org/wiki/Copper(I)_chloride
"Copper(I) chloride can also be prepared by reducing copper(II) chloride, e.g. with sulfur dioxide or a reducing sugar such as Ascorbic Acid (Vitamin C):
2 CuCl2 + SO2 + 2 H2O → 2 CuCl + H2SO4 + 2 HCl
2 CuCl2 + C6H8O6 →2 CuCl + 2HCl + C6H6O6
Many other reducing agents can be used.[14]"
|
My thought is that was that ascorbic acid is a reducing sugar because it has the ability to change oxidation states from dehydroascorbic acid
<--> ascorbic acid. It's a simple hydrogen/carbon/oxygen chemical. Glucose is very similar. From what I can see from wikipedia, The important
part of ascorbic acid is the hydroxil groups on the ring structure of the reducing sugar which participates in oxidation and reduction. They aren't
even part of an aldehyde group.
Glucose can form a ring structure with no aldehyde, or it can temporarily open and present a very active aldehyde group. So, Glucose has both the
necessary hydroxil groups to act the same as ascorbic acid (AKA: a reducing sugar.) and the ability to act as an aldehyde. So its a hexose, and
ascorbic acid looks like a hexose as well; they both have 6 carbons, and 6 oxygens, They both even form a ring structure.
It's not like I can look the oxidation and reduction potentials of reducing sugars up in my oxidation/reduction tables. My college texts aren't that
comprehensive. As useful as wikipedia is ... it doesn't list a half cell potential or anything like that which I can use to compare (at least yet,
December 14th, 2017).
https://en.wikipedia.org/wiki/Vitamin_C
https://en.wikipedia.org/wiki/Glucose
How can I know which reducing sugars will work, and under what conditions?
What's the key point about these reducing sugars that is different? What pattern of atoms do I look for to find a reducing sugar "like" ascorbic
acid?
[Edited on 15-12-2017 by semiconductive]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
| Quote: |
How do I find out the strength of a reducing sugar, compared to Cl- , OH-, and HCO3- ?
More importantly, why would it need to remove Cl-, when the copper atoms are not oxidized with two chlorines in the first place, but only one?
|
You don't need to remove chloride. You can't compare the strength of a reducing agent to that of chloride, hydroxide and bicarbonate, since these are
not reducing agents. I have no idea what Chemetix meant by chloride being a strong anion- you're not pulling off a chloride; you're adding an
electron.
| Quote: | I only added enough HCl to attack half the basic copper carbonate bonds; Did I miscalculate the molar concentration of HCl? Would you check for me?
Basic copper carbonate is in the Cu+2 oxidation state. The +2 is caused by BOTH hydroxil anion, and a carbonate anion. I didn't supply enough HCl to
replace all the CO2 and OH. Therefore, one of the bonds must still be attached to the copper ... because the copper did all dissolve into solution
once the acid was added; I don't think I created a solution where chloride attacked only some of the basic copper carbonate, and left part of it
alone. Otherwise, why did all the copper carbonate go into solution? |
If it all went into solution, then you neutralized all of the hydroxide, and probably converted a significant amount of the carbonate into
bicarbonate. But it's not the chloride that's attacking the basic copper carbonate; the chloride is a spectator ion. The hydrogen ions from the acid
are attacking the hydroxide and the carbonate.
| Quote: | | I assumed either the hydroxide or the carbonate would be easier to displace, and the copper atom would stay in the +2 oxidation state because one of
the anions was HCl, but the OTHER anion would still be either hydroxide or carbonate. |
An acid-base reaction will not change the oxidation state of copper(II).
| Quote: | | My hope was that a reducing sugar would have an advantage in a situation where chloride was NOT the only anion attached to the copper, but a weaker
anion was present. |
The chloride is *not* attached to the copper in dilute aqueous solution. It's just a counterion.
| Quote: | The Wikipedia article I was following to make copper I chloride explicitly said that reducing sugars (eg: ones LIKE ascorbic acid / vitamin C ) was
able to reduce copper II chloride to copper I chloride. So, they are saying a reducing sugar can even overcome chloride. But you say it can't?
Wikipedia doesn't specify under what conditions it happens. (eg: Do you have to dry out the solution, or does it precipitate, etc. )
|
CuCl isn't very soluble, so if the reaction occurs, it will precipitate out easily. Try it- if it doesn't go, heat it. Make sure the chloride ion
concentration is moderate (i.e., enough to make sure that your product will precipitate out, but not so high that you form soluble complex ions. Aim
for about 0.5 mol/L max).
[Edited on 15-12-2017 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by DraconicAcid  | | Quote: |
You don't need to remove chloride. You can't compare the strength of a reducing agent to that of chloride, hydroxide and bicarbonate, since these are
not reducing agents. I have no idea what Chemetix meant by chloride being a strong anion- you're not pulling off a chloride; you're adding an
electron.
|
|
DraconicAcid, your way of thinking is very narrow and restricted only to the solution.
For example, if I took table salt and dissolved it in water ... it's still called "salt water."
I know you are correct ... the chloride anions are not attached to the sodium when dissolved; however, when the water is evaporated, or the salt is
precipitated (by adding alcohol, or other chemical to physically change the solubility); the chloride ions would re-attach to the sodium and form
"salt" again. The chloride ions are not stripped (or displaced) from the sodium by alcohol or evaporation during the formation of crystals.
Two salts can also be added to water. For example sodium chloride, and copper acetate.
During the evaporation of water, two salts will form again. The particular salts that will form will be in proportion to the cation and anion
strength. I've done experiments like this, and unless the reaction is a "metastatis" reaction, the original two salts will precipitate out. In
general, if the evaporation is slow, distinct and tiny salt crystals will form at random locations in the flask; but each tiny crystal will be one or
the other kind of salt and relatively pure. The anions and cations in solution, during evaporation, do have a preference as to what chemical they
will bind with during solidification.
When I talked about "stripping" in earlier posts, I was thinking of the end result of the salt formed after evaporation or precipitation. I'm not
thinking about what is in solution.
People have a tendency to think about the end product. It's possible that's what Chemtix was thinking as well. It's not precise, but it's not good
to get hung up over that kind of language.
| Quote: |
If it all went into solution, then you neutralized all of the hydroxide, and probably converted a significant amount of the carbonate into
bicarbonate. But it's not the chloride that's attacking the basic copper carbonate; the chloride is a spectator ion. The hydrogen ions from the acid
are attacking the hydroxide and the carbonate. |
I didn't add enough HCl to neutralize all the hydroxide and carbonate.
Basic copper carbonate is: OH-Cu-CO3-Cu-OH
(note: It won't be spectator ion once evaporation or precipitation happens.)
The stoichometery of the first post was intended to add exactly the number of moles of HCl that I had moles of copper. Therefore, since the basic
copper carbonate has one hydroxide each, and 1/2 carbonate each, there isn't enough Hydrogen to make bicarbonate AND neutralize the hydroxide ions.
So, would you check the math of the first post. Because, your conjecture is impossible unless I made a gross math mistake.
The original chemical reaction mixture has been stirring at 55C for over two weeks. A very small amount of light orange colored precipitate did form
at the top surface of the sealed Erlenmeyer flask. No more oxygen can get in, but the orange precipitate has sunk to the bottom after about a week;
It's immeasurably small and not even a thick enough film to cover all the glass on the bottom of the flask. (Edit: This remark is slightly incorrect,
see photos in posts which immediately follow this one. The orange film is not enough to make the glass opaque.) The original carbonate was over two
millimeters thick covering the flask bottom before being dissolved.
If the carbonate is not displaced during evaporation/precipitaiton, (Eg: assuming the hydroxil is removed/neutralized more easily than carbonate.)
then I would expect: Cl-Cu-CO3-Cu-Cl to be the product of evaporation.
| Quote: |
| Quote: | | I assumed either the hydroxide or the carbonate would be easier to displace, and the copper atom would stay in the +2 oxidation state because one of
the anions was HCl, but the OTHER anion would still be either hydroxide or carbonate. |
An acid-base reaction will not change the oxidation state of copper(II).
|
Right, which is exactly what I just said.
| Quote: |
| Quote: | | My hope was that a reducing sugar would have an advantage in a situation where chloride was NOT the only anion attached to the copper, but a weaker
anion was present. |
The chloride is *not* attached to the copper in dilute aqueous solution. It's just a counterion.
|
But it will be attached when I remove the water; eg: if I put a vacuum pump on it and evaporate the water without allowing oxygen into the
solution. Note, I did say that wikipedia didn't tell me what conditions the reducing sugar had an effect in.
| Quote: |
CuCl isn't very soluble, so if the reaction occurs, it will precipitate out easily. Try it- if it doesn't go, heat it. Make sure the chloride ion
concentration is moderate (i.e., enough to make sure that your product will precipitate out, but not so high that you form soluble complex ions. Aim
for about 0.5 mol/L max). |
The glucose solution has already been baking for 12 days at 55*C. That temperature was chosen because another wikipedia article talked about brine
solutions changing copper I into a different compound at 60*C +. So, I chose a slightly lower temperature to not trigger a side reaction.
The number of moles of carbonate is ~0.008 dissolved in 50mL of water. So, double that number of moles of copper is present inside the carbonate:
~0.0016 mole /0.050L = 0.032 which is << 0.5 mol/L. So, your requirement is already met.
I could put ascorbic acid into another beaker, and I intend to do that. But doing the experiment isn't going to explain to me *WHY* ascorbic acid
( a reducing sugar ) works when glucose (another reducing sugar) doesn't.
eg: the original question about strength of the reducing sugar vs. carbonate or chloride could be rephrased: How do I compare the strength of the two
reducing sugars ?
I really don't see how it helps to think about what's going on in solution when I can't really test it. At some point, for reduction to have
happened; chloride ion is prevented from attacking the copper during evaporation because of the presence of an electron transfer, or a hydrogen atom
has reduced copper to the +1 state.
What matters is not so much "when" the reduction occurs; rather what I'm focused on is that Wikipedia says a reducing sugar can cause Copper I to be
made from copper II . Wikipedia was talking about a solution of CuCl2. In order for that to be reduced, at some point .. one of the chlorides had to
find something OTHER than copper to be attracted to during evaporation of water or during precipitation. (eg: Chloride finds hydrogen and turns into
a gas and evaporates.)
EDIT: In the footnotes of wikipedia, they cited an old text book which happens to be hosted by sciencemaddnes.org, but when I tried to access the
book, the PDF reader comes up, but the document never shows. Hence, I can't read the conditions of the original experiment with ascorbic acid.
[14] Glemser, O. and Sauer, H. (1963) "Copper(I) Chloride" in Handbook of Preparative Inorganic Chemistry, 2nd ed. G. Brauer (ed), Academic Press, NY.
Vol. 1. p. 1005.
http://www.sciencemadness.org/library/books/brauer_ocr.pdf
I would appreciate someone checking the original book for me and telling me the conditions of the experiment that wikipedia is citing.
[Edited on 17-12-2017 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by Boffis  | | If you have time the simplest method is to place bare copper wire or bits of copper in a jar and fill it up with dilute ammonium chloride solution and
seal tightly. |
I bought the two dram vials from e-bay (~50cents/piece). I thought I had purchased the borosilicate glass ones, but now that I check the listing,
it doesn't specify the kind of glass; (seller: homeluxe-grandparfumes ,8mL 1/4Oz Empty Glass Bottle Screw Top Clear Sample Vial Perfume Oil 2 Dram )
So I bought the wrong listing, accidentally. I'll heat one later today to check the coefficient of expansion of the glass and see if it's lime glass
or borosilicate; but I'm sure it has a phenolic cap. Phenolic caps can handle up to 220*C, so it's a pretty decent vial for doing experiments in.
I'm curious about what kind of container you did the experiment in.
I measured my vials dimensions at 21.5C for the glass (not the cap). There is a photo of that + an ABS pipe I drilled to hold 12 vials with the
insides of two of the caps exposed:
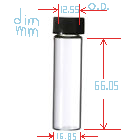 
It should have more than 8mL of storage, eg: around 11 to 14, depending on glass thickness. I can't quite measure the inside. I will probably measure
the volume by weighing water later today.
As you can clearly see, some of the phenolic caps come with a foam sealing liner, but some of them don't. The manufacturer is kind of lazy and
inconsistent.
When you did your experiment, what kind of container did you seal the chemicals in; and was it an air-tight seal with some way to exclude air bubbles?
eg: Did you do anything to make sure the chemicals were in an inert environment? eg: teflon, etc? and, especially, Do you have any idea of how much
wire and ammonium chloride that you used?
Also, was the container exposed to light; or was it kept in a dark, even temperature place (crystal growth is often affected by light or thermal
gradients. )
[Edited on 17-12-2017 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Original experiment photo update.
It's been two weeks. Here is a photo shoot showing the Dec 16,2017 state of the glucose and copper carbonate/HCl solution described in the first post
of this thread. The first picture, is a copy of the last picture in the opening post of the thread so you can compare colors. All pictures are shot
with the same cell phone camera. warning: To my eyes, the actual color of the solutions was slightly more green than the computer or cellphone screen
shows. The CCD camera in the phone tends to emphasize blue over green; still,all photos are with the same phone, so the differences in shades
between photos is accurate relative to the other photo.
  
As you can see, the solution color has turned much greener over the last 14 days. A very fine orange colored film is adhering to the glass of the
flask. At first, I thought it was just on the surface of the solution, and had sunk to the bottom of the flask. But now, since I took the flask off
the magnetic stir plate, I can clearly see the orange film adhering to the glass of the flask and being reflected by the glass on the bottom of the
flask. That film could easily be either copper metal, or something like copper I oxide. Cu-O-Cu. It's hard to tell. There was a tiny bit of the
film floating on top of the solution several days ago, but it's no longer there. The film appears to be entirely coating the glass.
It's important to note that the air head-space of the Erlenmeyer flask is sealed under a glass plug. The plug has not been removed at any time in
the last two weeks, so only the air in the head-space could have supplied any oxygen, and it is very limited in quantity. I also have reducing sugar
in the solution, so hopefully the dissolved oxygen is not really causing oxidation.
The only other unusual issue is that the teflon stir bar (not clearly visible in pictures) has a black spot on the part that rubs against the glass
on the bottom of the flask. When I shook it and got the stir bar to flip over, the black mark on top of the bar dissolved while another black mark
formed on the bottom of the bar. I have no idea what would cause that.
I set the constant temperature at 55*C, and covered the flask with refractory since the experiment began. The original refractory brick chunks were
not very effective in keeping the entire flask at the same temperature as the hot plate. I can't put a thermometer in the flask without introducing a
stainless steel rod, or leaking air. So, the early part of the experiment's liquid had an average temperature below 55*C. The temperature probe is
actually measuring the hot plate outside the flask, just next to the flask.
I'm lifting a new insulation jacket in the photo. It's made of cheap (disposable) plaster of Paris and pearlite. I will talk about improving that
more in another thread, as my first casting isn't very professional. In other experiments I've had it weaken, crumble and crack when heated above 150C
in a kitchen oven. However, when kiln heat like like 700C to 900C is applied, plaster of Paris can become really hard and tough after shrinking.
It's fickle stuff to make molds and shaped objects from that can take heat consistently ... I'd like to fix that. At least, plaster/pearlite is a
much better insulator than aluminum foil which is typically shown by home chemists on youtube.com to wrap flasks during heating for distillation.
[Edited on 17-12-2017 by semiconductive]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. I'm not very careful, but when I mix CuSO4 solution and Ascorbic Acid, and return a week or so later, I find nano particles of copper, and a
clear solution. That's it. Now, if there is Cu 1+ remaining in the solution, I haven't checked.
Got experimental details, from some folks that claim to have the Cu1+ thing..... pretty well wired.
https://www.dimanregional.org/site/handlers/filedownload.ash...
[Edited on 18-12-2017 by zed]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by zed  | | Ummm. I'm not very careful, but when I mix CuSO4 solution and Ascorbic Acid, and return a week or so later, I find nano particles of copper, and a
clear solution. That's it. Now, if there is Cu 1+ remaining in the solution, I haven't checked. |
There won't be. You need an anion that forms an insoluble compound with copper(I) to stabilize it- chloride or bromide, for example.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
2
3 |
|