Lurker
Harmless

Posts: 11
Registered: 6-11-2017
Member Is Offline
Mood: Static
|
|
Learning Chemistry from Scratch: Books and Advice
I live in a third-world country where education is a terrible joke, so I wasn't taught chemistry in school and now I've taken upon myself to learn it
from scratch. I'm saving some money so I can
a) Purchase books;
b) Eventually set up a home lab.
I want to design a curriculum that will take me from virtually knowing nothing about chemistry to a college-level chemistry.
I have spent time researching the web and this particular forum, and I got some recommendations, but I figured I'd focus my search by
starting this topic.
Can you please recommend books that would help accomplishing my goal of going from beginner to "well-rounded and somewhat advanced" by myself?
(I already have Robert Bruce Thompson's Illustrated Guide to Home Chemistry Experiments, which I've found fascinating; it will be quite
useful when I get my lab. But of course I'll need much more reading material, so here I am.)
Finally, based on your experience learning chemistry, is there any general advice you can give regarding my project?
Thank you very much.
"The man who never alters his opinion is like standing water, and breeds reptiles of the mind." — William Blake
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
http://www.chemguide.co.uk/ is about as good as you are going to get as far as building up basics in a systematic fashion. It is intended as a
study /revision guide to prepare for exams but its explanations are clear enough that it can stand in for a textbook. And it is probably better than
many.
Doing more than just lurking on this site will do wonders too. You might want to consider a name change and active involvement.
Getting a lab is a wonderful idea and it does not need to be too expensive or too complex -- at least at the beginning. I recommend building it
project by project. You ill be amazed at what can be accomplished using OTC supermarket items and a few jam jars.
In terms of building a lab the two most significant hurdles that are encountered early on are a good supply of acids and a good DC power supply (with
suitable electrodes). I am sure that many here can give you a suggested lab inventory but a whole lot depends on what kind of chemistry you intend to
pursue, what scale you intend to work at and what resources you have easily available.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
This might be a useful resource for you. its a open book library, https://open.umn.edu/opentextbooks/BookDetail.aspx?bookId=69
As for labs etc, remember safety gear before fancy glassware. No point having chemicals like Sodium Hydroxide or strong acids and no eye protection,
to me eye protection is the first thing you get when starting a lab. Gloves are good depending on what your using.
As a rule your safety equipment should be ahead of the type of experiments you do. Working down wind outside when making say Nitric Acid, is all fine
and dandy, but while a change in wind might not kill you, that splash of nitric acid outside with no eye protection could really ruin your day.
Dont jump in the deep end, and always ask for info if your not sure on a safety question. General rule though, if its so dangerous and you need to ask
the most basic questions, your working way above your ability. There is a library here at SM, its full of the classics.
Once you get your feet wet there are 'other' resources around  . .
Dont buy cheap second hand books off ebay, generally those have my name on  .
Dont buy cheap books off abe books for the same reason lol .
Dont buy cheap books off abe books for the same reason lol
[Edited on 8-11-2017 by NEMO-Chemistry]
|
|
|
Lurker
Harmless

Posts: 11
Registered: 6-11-2017
Member Is Offline
Mood: Static
|
|
@j_sum1: Right now I'm stuck with survival considerations, so I can't participate here often (even because, not knowing the least of chemistry yet, I
don't have much to offer). I'll save the name change and posting more often for when my life stabilizes a bit and I start a organized study routine
and begin to get a lab together — the plan is following Robert Bruce Thompson's Illustrated Guide to Home Chemistry Experiments regarding
what to buy and what will be my first experiments. Thank you very much for your reply and the recommendations.
@NEMO-Chemistry: I'm taking my time, I don't intend to set up the lab until I'm able to do it properly; it will take some time. Thank you very much
for the link and for the precious advice; I intend to be very careful (after all, underestimating something as nitric acid could ruin more than my
day). Oh, as for the books, supposing they won't cost more for having your name, I'd be glad to "catch 'em all." 
"The man who never alters his opinion is like standing water, and breeds reptiles of the mind." — William Blake
|
|
|
diggafromdover
Hazard to Self
 
Posts: 84
Registered: 24-2-2015
Location: New Hampshire
Member Is Offline
Mood: Inconherent
|
|
I find this book a very interesting read:
https://www.amazon.com/Organic-Chem-Lab-Survival-Manual/dp/1...
Enjoying second childhood with REAL chemistry set.
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
If you are serious about study the References forum here is an excellent resource. PM admin for access
/CJ
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Learning chemistry does not require a fully equipped Lab, nor will every experiment succeed if you have one.
'Doing it Properly' does not exist. There is just 'Doing It' and learning.
Get a couple of empty plastic bottles and a potato - extract some starch from it.
This might not sound exciting, but the experience is very useful on many levels, not least of which is that iodine titration is more easily done with
potato starch, not so easy if all you have is lots of fancy equipment and no starch.
Doing that would also give you real Experience of plant extraction, and the relative insolubility of starch at RT, which no book can truly give you.
Try it and see.
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
In my opinion the Zubrick text is overkill for a beginner (I admit I would like one myself but not for $50). The Thompson book is very good for a
beginner. For a textbook for an absolute beginner I would look online for a secondary school introductory level book. I believe the best way to get
started is to work through a qualitative analytical chemistry text, performing the typical reactions of the various ions on a small scale, which means
generally simple equipment and small amounts of chemicals needed. Best of luck in your chemistry endeavors.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Lurker  | @j_sum1: Right now I'm stuck with survival considerations, so I can't participate here often (even because, not knowing the least of chemistry yet, I
don't have much to offer). I'll save the name change and posting more often for when my life stabilizes a bit and I start a organized study routine
and begin to get a lab together — the plan is following Robert Bruce Thompson's Illustrated Guide to Home Chemistry Experiments regarding
what to buy and what will be my first experiments. Thank you very much for your reply and the recommendations.
@NEMO-Chemistry: I'm taking my time, I don't intend to set up the lab until I'm able to do it properly; it will take some time. Thank you very much
for the link and for the precious advice; I intend to be very careful (after all, underestimating something as nitric acid could ruin more than my
day). Oh, as for the books, supposing they won't cost more for having your name, I'd be glad to "catch 'em all."  |
Access to the ref section as mentioned is absolutely one the best things you can do.
For some reason not everyone finds this straight away (myself included), but SM has a library, its got MANY great books, the link is here
http://library.sciencemadness.org/library/index.html
The one downside with the ref section is download size, you cant download/upload more than 8mb, this can be an issue with books.
I am a avid reader, i tend to pdf books first, then once I find one i really like I hunt for a hard back copy.
There are a number of sources for books in pdf format. I am unsure what level you want, but working on the principle that as you learn your needs will
increase, i can send you some pdf books if you like.
PM me your email or if you have a low limit on email attachments, pm me anyway, i have another way to get books and papers to you.
Over the last two years I have amassed a huge amount of both pdf books and paper books. I cant scan the paper ones, but if you find a title of a book
you cant get via the usual pdf way, pm me. I might already have a copy.
Someone on here told me about some software to organize your papers and books. Grab it now while your collection is small!
Its called mendeley
Download the desktop version here
https://www.mendeley.com/downloads
its free but before you sign up come back and let us know you installed it. The reason is, mendeley also puts your collection online, this is free
upto a certain size.
What I didnt know however, menedely dosnt have to archive all your collection online to be used. So before you go adding your folders etc let us know,
i will post details of how to stop it uploading all your books and papers, that way you can choose those you want access to from the cloud.
It dosnt mean you cant have your entire collection organized in mendeley, because you can, but you have to tweak the settings first.
there are other open source version of this kind of software, so far having tried a few, i like mendeley the best. Its transformed my ability to find
books and papers on my hard drive!!
Really worth getting and installing the free version.
I will pm you a resource for pdf books, its not the normal one, it does however have titles on it that the more frequently used service dosnt.
Are you after general chem books, organic chem, or inorganic chem? or a mix?
If I can help then give me a shout. Good luck and trust me......Chemistry is alot of fun. Also keep in mind what aga said, you can do alot and have
alot of fun for little money.
The point I was trying to make was stay within your safety limits. But also remember Chemistry is alot better if it isnt a spectator sport, or put
another way...........
its more fun doing than reading, but personally i like to read and understand before I do.
Another good resource is this journal
http://pubs.acs.org/loi/jceda8
Be aware it has alot of experiments for all abilities, have a good browse through back issues. The trick to it is find the stuff you like via the
abstract.
Then ask for a copy of the paper in refs section, use the article DOI number and or full title though, pref with a link

Be aware the journal is slightly annoying, some the experiments have additional information available, most times you dont need it, but while most can
get you the full paper, its harder to get the additional info unless your subscribed.
[Edited on 9-11-2017 by NEMO-Chemistry]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have the organic chem survival book, but i dont think its the tenth ed. pm me your email if you want a pdf copy
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
What do you mean by "from scratch" - you mean learn chemistry with no prior knowledge of chemistry?
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JJay  | | What do you mean by "from scratch" - you mean learn chemistry with no prior knowledge of chemistry? |
According to his first post at the top, thats exactly how i read it. He mentions being in a third world country, so I think his resources might be
limited. Creative OTC looks to be the key 
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Every time I see this question I mention stoichiometry. It is something you really should know about as a chemist. Pretty much any introductory
chemistry book should cover it.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
country would help, that way text books that schools use in your location could be advised on.
@JJay
Its hard to tell from the first post what knowledge he has, he mentions 'above what he has self learned', but dosnt say exactly what that covers...
I have UK GCSE and A level chemistry texts used in schools in the UK. these cover the basics but in a crap way. The site I linked too also has alot of
basic info in.
Stoichiometry is only of use if he knows how to work out basic formula.
So maybe it would be an idea if the OP tells us what level he is actually at.
|
|
|
Lurker
Harmless

Posts: 11
Registered: 6-11-2017
Member Is Offline
Mood: Static
|
|
@diggafromdover: I've seen a lot of recommendations of Zubrick's book here on the forum. It's in my list. Thank you very much.
@Corrosive Joeseph: Will do. Thank you!
@aga: By "doing it properly" I meant waiting until I have a place where I can experiment and a better income source—like I wrote above, I'm dealing
with basic survival right now. I won't be just waiting, though, I have a plan. Anyway, your recommendation of going for it is very good, I won't
forget it. Thank you very much.
@CharlieA: "You never know what is enough unless you know what is more than enough," wrote William Blake; a little bit of overkill might not hurt too
much. In any case, I've got some suggestions for my starting textbook: Chemistry: The Central Science (Brown, Lemay, Burstein),
Chemistry (Steven S. Zumdahl and Susan A. Zumdahl) and Chemistry: A Molecular Approach (Nivaldo J. Tro). It will be a while before
I'm able to start buying any books, so I have time to decide. Your suggestion is akin to what I intend to do then. Thank you very much for your post.
@NEMO-Chemistry: I'll ask an admin about the access to the reference section, but I've already discovered (and "checked out" some books from) the
forum's library. I'm an avid reader like you—my money goes for books then food; I've also amassed enough digital content for many lifetimes. As for
chemistry books, I'm not focusing on a single area. And by the way, I'm downloading Mendeley to see if I can manage to install here. Like you, I
intend to read enough to understanding what I'm doing before I set to actually doing it. Than you a lot for your post and your offered help. I'll U2U
you shortly.
@JJay: Exactly. No prior knowledge whatsoever. I can't start focusing on chemistry right now, so I haven't started studying it yet; at the moment I'm
collecting material and planning about what to acquire later. So I meant it, my knowledge of chemistry is currently null, but I won't forget about
stoichiometry when I begin. Thank you very much for your comments.
"The man who never alters his opinion is like standing water, and breeds reptiles of the mind." — William Blake
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
If you have some Aim in mind, now would be the right time to tell people.
It is very hard to suggest anything as 'Chemistry' is so large.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
as you have alot of digital information, mendeley will save you a great deal of time! Once your used to it its great.
|
|
|
Lurker
Harmless

Posts: 11
Registered: 6-11-2017
Member Is Offline
Mood: Static
|
|
@aga: My first goal is to study general chemistry before breaching to any specific areas. I don't know enough to actually know the options right now,
which is why my posts may seen vague.
@NEMO-Chemistry: Let's see how I adapt to it. 
"The man who never alters his opinion is like standing water, and breeds reptiles of the mind." — William Blake
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
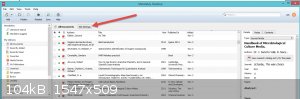
First job with mendeley is open free account. then look at first pic.
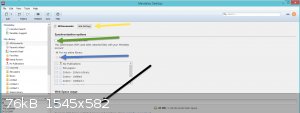
On the Lib screen in the software, press the settings button arrowed and that will take you too pic2.
uncheck the box with the green arrow
ignore the blue arrow for a min
the black arrow shows you how much online space you used, having unchecked the green arrow however, means that you are not dumping all your files
online.
Put your papers and books into a couple of folders, or be really neat and use a folder with a subfolder structure.
then use the watch folder button in the software to choose the folders where you save your different files. I have a special folder where I keep files
i might want while I am away or whatever.
When that happens I check the blue arrow box and just choose the folder i want to sync in the box below the blue arrow.
Once your happy click the yellow arrow to get back to the main screen. the sync button on the main screen syncs the folders you chose to copy online.
Books you add will normally show up a little while after you put them in the folder, or sometimes you just need to close and reopen the software. Do
not exceed the black arrow limit or they ask you for money.
There are loads of vids online for the finer points, but the above will get you started without smashing your online file limit.
You can store as many files as you like on your system and it will keep them organized. So i pick carefully the folders where I actually want internet
access to some files.
Also it has a academic suggestion thing, but i will leave you to discover the delights the software offers.
Oh and use the tags feature as well. Trust me there is loads of stuff to learn with it, but that is the very basics.
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
I second the motion of Chemistry: The Central Science for a general chemistry text. I bought mine used on ebay a couple of years ago, and am using it
to refresh all of the chemistry I have forgotten since graduating from college in 1962. Keep at it, I think that you are on the right track.
|
|
|
WangleSpong5000
Hazard to Others
  
Posts: 129
Registered: 3-11-2017
Location: Oz
Member Is Offline
Mood: Curious
|
|
Quote: Originally posted by CharlieA  | | I second the motion of Chemistry: The Central Science for a general chemistry text. I bought mine used on ebay a couple of years ago, and am using it
to refresh all of the chemistry I have forgotten since graduating from college in 1962. Keep at it, I think that you are on the right track.
|
I third this motion. It's required reading for Ist year undergrads in Oz doing a BSc and even more so if you are doing said degree in Applied
chemistry. There's a brilliant O Chem text I would highly advice also but that's for later... fundamentals first...
Stuff you MUST know:
The periodic table is so much more than it seems at first glance. It can tell you more that you know.
The basics:
What are atoms, molecules, elements, compounds etc. and the protons, neutrons and electrons etc. that they are consisted of. How these particles
interact.
Bondng types: covalent, ionic, metallic (and later on the deeper aspects of why things bond those ways)
Basic electron valence theory and rules behind it.
IonIc compounds, ions and ion charges (cations (+), anions (-)...), common polyatomic ions ect.
Different reaction types and how to balance them: combustion reactions, precipitation reactions of ionic compounds, acid/base reactions
The concept of acids and bases and relevant reactions
The concept of the mol and what AMU's are, Stoichiometry, what it is, how and and why we use it. HINT: it's basically just fiddly aritmetic that
appears ALOT harder than it actually is.
|
|
|
Lurker
Harmless

Posts: 11
Registered: 6-11-2017
Member Is Offline
Mood: Static
|
|
@NEMO-Chemistry: It's been some years since I started using text files to organize; I'll try to install Mendeley here and study it, using your post as
reference. Thank you very much!
@CharlieA: It certainly seems to be a very good book! Thank you!
@WangleSpong5000: Welcome to the forum! I really appreciate your mapping what I'll study in the future. Good luck on your studies and thank you very
much for your post.
"The man who never alters his opinion is like standing water, and breeds reptiles of the mind." — William Blake
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Oh, if you want to know specific values of basically anything, http://www.wolfram-alpha.com is very helpful. You can type in "mass of 50 mmol of phenyldecanol", for example, and it will spit out the value immediately. (I chose that substance because its molar mass is
obvious from its name, but would still require manual calculation otherwise, since it's probably not useful or common enough to be able to look it up)
Google will do some of this stuff too (I used to amuse myself by typing "speed of light in cubits per fortnight" into google. For some reason I used
to think doing that was hilarious.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Your welcome, someone on here gave me the heads up on the software, having had a nightmare for several years finding files, its been extremely useful.
I nearly didnt use it because of the online limit, but once you discover how to bypass that  alls good. alls good.
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
Look up 'electronegativity'. You can find charts that list the electronegativity of the different elements, which is a number in the 0-4 range.
It's a way of describing how badly that atom wants electrons. If you look at chlorine, it has an electronegativity of around 3. Sodium metal, on
the other hand, has an electronegativity of about 1. The chlorine wants electrons much, much more than sodium does. If you put these two elements
together in a flask, they will react violently; the more electron-hungry chlorine will grab an electron off the sodium, forming Cl- ions (which now
have an 'extra' electron) and Na+ ions (which have lost an electron.)
Grasping this one basic idea (that atoms have different levels of willingness to gain or give up electrons) will go a long way to understanding why
things react (and how.)
To extend the idea, water has an oxygen atom (electronegativity of about 3.5) and two hydrogen atoms (EN of 2.2). Because the oxygen atom is
'stronger', it will pull electrons towards itself, making itself more negatively charged, while making the hydrogen atoms more positively charged.
Because of that, water has positive and negative ends to it, just like a magnet. That makes water molecules stick together fairly tightly, like a big
bag of magnets all stuck together.
And that's why it takes so much heat to boil water and rip those water molecules apart from each other.
This basic idea of atoms fighting over electrons (and the battle producing different levels of positive and negative charges in molecules) is pretty
much the foundation of chemistry, so keep it in mind when you look at simple reactions (like acids and bases neutralizing each other.)
|
|
|