theswitch
Harmless

Posts: 2
Registered: 18-1-2017
Member Is Offline
Mood: No Mood
|
|
alpha-iodo propiophenone
on rhodium the synthesis is carryd out with bromine and glacial acetic acid,
but can iodine be used for halogenation of propiophenone, I expect it would
be allot less messy. Should one use acetic acid or another solvent ? It is
more expencive ,, but I dont want to mess with bromine.
|
|
|
Texium
|
Thread Moved
24-1-2017 at 07:11 |
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I believe that alpha-iodination with I2 is best accomplished with copper(II) salts or CAN as catalyst. An example of this would be the use
of I2 with Cu(OAc)2 in AcOH at 60*C. The copper(II) salt acts as catalyst to convert the iodine into the reactive iodonium ion,
as a base to neutralize hydrogen iodide, and reoxidizes iodide to iodine. There is also the reaction of a ketone with I2 in
1,2-dimethoxyethane (DME).
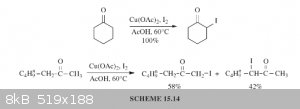
https://books.google.com/books?id=tWrIBAAAQBAJ&lpg=PA256...
There are other reagents that can be used to generate Br2 in-situ that keeps you from having to handle it directly, such as NaBr/Oxone,
DBDMH, CuBr2, and NBS. I have used NaBr/Oxone, DBDMH, and NBS for alpha-bromination with great success. I really recommend the usage of DBDMH in AcOH
with H2SO4/p-TsOH as catalysts.
[Edited on 24-1-2017 by Loptr]
[Edited on 24-1-2017 by Loptr]
[Edited on 24-1-2017 by Loptr]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Depending on what you're after, alkyl nitrites can react with ketones at the alpha position too, to give oximes, which are much more stable than their
imine counterparts. They can also be reduced to amines directly:
http://orgsyn.org/demo.aspx?prep=cv2p0363
[Edited on 1/25/17 by Melgar]
|
|
|
laserlisa
Hazard to Self
 
Posts: 52
Registered: 5-2-2016
Member Is Offline
Mood: No Mood
|
|
NBS in acetonitrile with TsOH as catalyst is reliable and high yieldning for alpha-bromination imo.
If you for some reason want the iodopropiophenone you can do a finkelstein swap using NaI after the bromination.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I was thinking about making this iodoketone as well, considered the many options, including a finkelstein with NBS to obtain NIS.
But now I am just thinking, iodination of a ketone is way more complex than it seems to be with a simple bromination, I will just run a finkelstein in
acetone on the bromoketone and precipitate the iodoketone afterwards with water to avoid distilling the acetone off afterwards.
Edit: I have to say, I once made a iodoketone, using I2 in DMSO on methyl indolyl ketone, which was giving a nice yield and was a pretty quick
reaction.
Surely this works on propiophenone as well.
[Edited on 19-12-2019 by karlos³]
|
|
|
dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
i am thiniking from some time abaut that reaction too, i have an document abaut a-iodination of acetophenone wich i will attach, the most promising
method i see is I2/CuO in Methanol reflux or I2 in DME, but what shoud be the reaction conditions, firstly disolve the I2 with CuO in Methanol and
drip that over propiophenone or whats the best way to do it, since I2 i think wont disolve in methanol easy, or woud I2 be disolved more easily in DME
and that solution be refluxed with propiophenone and the paper states abaut exess iodine used, what I2 exess is needed and what woud lead to
diiodination, since TLC isnt an option or any monitoring device, what reaction time shoud be expected with these 2 methods ?
Attachment: Iodine-Copper Green Iodination.pdf (540kB)
This file has been downloaded 508 times
[Edited on 19-12-2019 by dextro88]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
The reaction conditions are clearly described in the paper, and of course does iodine dissolve very easily in methanol.
The ratios of reagents are also clearly stated in there, you just need to calculate them for your substrate.
|
|
|
dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
"Under optimized conditions, treatment of p-methoxyacetophenone (1a) with 1.0 molar equivalent iodine and
1.0 molar equivalent finely powdered copper(II) oxide
in methanol at reflux for one hour afforded the corresponding a-monoiodo ketone 1b in 99% yield"
i think the case is solved 
Attachment: a-iodination.pdf (103kB)
This file has been downloaded 561 times
[Edited on 19-12-2019 by dextro88]
|
|
|