Kyryllium
Harmless

Posts: 5
Registered: 3-7-2016
Member Is Offline
Mood: No Mood
|
|
Hello, i am new here!
I am really new to chemistry, i know basic stuff like elements and such, but i don't know how combinations work, or how to calculate reactions etc.
Are there any really good books to get me started? i'm not looking for an experiment book, i'm looking for something to study with, something that
explains equations... I can't really find anything, could anyone please recommend a lecture for me?
Thanks in advance
Kyryllium
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It sounds like you should start with a good high school level chemistry textbook. There are many of them out there, and they're all fairly similar.
You'll need something like that to get the basics. I'll admit, it's not the most fun reading, but once you understand it you're free to move onto
bigger and better things.
Welcome to the forum!
|
|
|
Trevor9424
Harmless

Posts: 26
Registered: 13-5-2016
Location: United States
Member Is Offline
Mood: Corroded
|
|
Perhaps a good start would be the reactivity series.
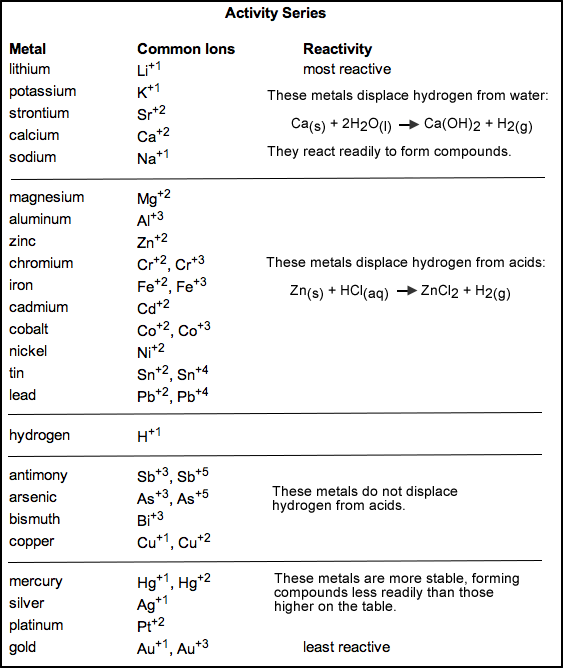
The elements near the top are more reactive than elements near the bottom of the chart. For example, copper in a copper salt, such as copper sulfate,
is less reactive than iron so iron metal can replace copper in the salt to create an iron salt such iron sulfate and copper metal.
CuSO4 + Fe ---> FeSO4 + Cu
In a more exact equation, iron reduces copper in copper sulfate to copper metal and iron is oxidized to iron sulfate.
Cu2+ + Fe ---> Cu + Fe2+
For a different example, if you added silver to copper sulfate solution, no reaction would happen because silver is less reactive than copper.
Try to get your hands on some transition metal salts (such things can be bought on sites in 30 gram or less amounts such as on Home Science Tools) and
experiment around in reference to the reactivity series. Good luck and welcome!
[Edited on 7-3-2016 by Trevor9424]
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
I like Chemistry: the Central Science. It is a college-level textbook, but it doesn't assume the reader has any prior chemistry knowledge. I got mine
one edition older than the current edition (12 vs. 13) and I got it used, so it cost less than $25. I do strongly recommend you do the problems at the
end of each chapter - only practice will make the info stick in your brain. The book itself has the answers to about half the problems (in black ink).
I also bought the separate answer book in order to have the answers to the red ink problems too, but you don't have to go that far.
|
|
|
TinSandwich
Harmless

Posts: 29
Registered: 24-2-2016
Member Is Offline
Mood: No Mood
|
|
Wikibooks has a free chemistry textbook, it's basic but good. It also is very straightforward and straight to the point. Here: https://en.m.wikibooks.org/wiki/Introductory_Chemistry_Onlin...
|
|
|
Kyryllium
Harmless

Posts: 5
Registered: 3-7-2016
Member Is Offline
Mood: No Mood
|
|
Thanks a lot!
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
I found Khan Academy to be helpful, https://www.khanacademy.org/science/chemistry
|
|
|
Kyryllium
Harmless

Posts: 5
Registered: 3-7-2016
Member Is Offline
Mood: No Mood
|
|
Thanks a lot!
|
|
|