| Pages:
1
2 |
Korialstrasz69
Harmless

Posts: 34
Registered: 23-9-2015
Member Is Offline
Mood: No Mood
|
|
Vanillin to Piperonal
I found another way that makes perfect sense to me at least,but i know it's wrong and i want to know why,i am still a beginer in organic chemistry
Most reactions involve demethylating of Vanillin followed by remythelation by Williamson Ether Synthesis i think.
Point is,why the fuck demethylate and then methylate again ? Just free radical halogenate !
Of what i know is, the alcohol group wont be effected byfree radical bromine ir by hydrobromic acid, And the ring is resistant to halogination without
a catalyst i suppose,which leaves us with the methyl and the carbonyl groups to be halogenated.By slowly adding bromine to vanillin while hv ight is
directed through the apparatus bromine will homolise brominating the methyl group's first hydrogen,which should rect immediatly with the alcohol group
through a Williamson Ether Synthesis reaction,closing a ring.
Further bromination will occure to both the methyl and carbonyl group (I suppose).
So after that the product is dehalogenated,i know things can be dehaligenated but i don't exactly know how really
I also know know that c-Br bond is the weakest bond possible since iodine wont work here
So if the bone Br-c is weak and C-H is strong shouldn't it be fairly easy to debrominate the product back into piperonal ?
That's pretty much it.
And hey sorry about a few spelling errors and whatnot english isn't my first language
And no am making no drugs i don't even have a lab i am just discovering the beauty of orgnic synthesis and thanks ahead ya'll
|
|
|
chemplayer..
Awesome

Posts: 48
Registered: 12-2-2016
Member Is Offline
Mood: No Mood
|
|
Having done lots of experiments with vanillin, I can tell you that the ring is extremely reactive as it's a phenol and very succeptible to
electrophilic substitution. Iodine in KI solution will substitute, as will dilute nitric acid. What you are thinking of will work for relatively
unreactive rings such as toluene but in this case you're going to run into difficulties.
Due to it being a p-phenol the ring is also fairly easily oxidised and so anything like chlorine stands a reasonable chance of oxidising this as well
I would guess, as well as potentially oxidising the aldehyde group to the carboxylic acid.
Demethylation is a pain. We've tried about 6 methods so far, all of which need quite difficult reagents to obtain and none have given us more than
about a 10% yield, even with really stringent conditions and drying. There may be a sneaky way but we haven't found it.
That said, don't be discouraged as vanillin itself is easily available, very interesting and you can do a lot of great experiments with it
(condensations, benzylidene compounds, nucleophilic substitution on the carbonyl, electrophilic substitution etc.).
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Aw. Vanillin may be difficult to de-methylate. Ethyl Vanillin....not so much.
Probe via the search engine. Most problems have been broached, and solved, previously.
Also of note....There may be difficulties de-methylating vanillin via a strong base, as aldehydes don't like that. Eugenol however, might be cleaved
in such a manner. Of course, it may isomerize to the Iso-propenyl form, during de-methylation, but this might be of little consequence.
As allyl and isopropenyl isomers, may react similarly in some popular reaction sequences.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Quote: Originally posted by zed  | Eugenol however, might be cleaved in such a manner. Of course, it may isomerize to the Iso-propenyl form, during de-methylation, but this might be
of little consequence.
As allyl and isopropenyl isomers, may react similarly in some popular reaction sequences. |
Indeed so! Or get an essential oil and isomerize it with base. If you need a small q. of piperonal u2u me. I have rather more than I would need for
the next 10 years.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by chemplayer..  | | Having done lots of experiments with vanillin, I can tell you that the ring is extremely reactive as it's a phenol and very succeptible to
electrophilic substitution. Iodine in KI solution will substitute, as will dilute nitric acid. |
Hmmm...I wonder if you could do a one-pot transformation of vanillic to nitrovanillic acid?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
chemplayer..
Awesome

Posts: 48
Registered: 12-2-2016
Member Is Offline
Mood: No Mood
|
|
Yes, heating vanillin with KOH in the molten state gives protocatechuic acid (demethylated, but converted into the carboxylic acid). So you can
demethylate using strong base but the aldehyde will certainly react.
And nitration of vanillin to 5-nitrovanillin is easy but does need the right conditions for a quality product to be obtained. (We just did a video on
this last week in fact!) I'm guessing it would work likewise for vanillic acid too.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by chemplayer..  | Yes, heating vanillin with KOH in the molten state gives protocatechuic acid (demethylated, but converted into the carboxylic acid). So you can
demethylate using strong base but the aldehyde will certainly react.
|
A procedure for any interested parties: http://www.orgsyn.org/Content/pdfs/procedures/CV3P0745.pdf
|
|
|
Korialstrasz69
Harmless

Posts: 34
Registered: 23-9-2015
Member Is Offline
Mood: No Mood
|
|
First thanks a whole lot
And about demythelation you're the pro and you seemed to try so many way but just to make sure have you tried AlBr3/nitrobenzene method ? I don't have
a lab and never tried it before but it seems like the reagents are easy to aquire for chemists like you,although hazardous.
Looking forward for your video about it lads and thanks again !
|
|
|
chemplayer..
Awesome

Posts: 48
Registered: 12-2-2016
Member Is Offline
Mood: No Mood
|
|
Definitely not pros! But we haven't tried that method no ; nitrobenzene in large quantities (as a solvent) is pretty toxic and although we have access
to AlCl3 and AlI3, AlBr3 is going to be a bit more difficult to prepare in practise due to the extremely exothermic reaction between iodine and
aluminium. It could be one for us to try at a future date on a small scale perhaps.
|
|
|
Korialstrasz69
Harmless

Posts: 34
Registered: 23-9-2015
Member Is Offline
Mood: No Mood
|
|
Whoa thanks god I don't do chemistry I would've died long ago
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
"Quote: Originally posted by chemplayer..
Yes, heating vanillin with KOH in the molten state gives protocatechuic acid (demethylated, but converted into the carboxylic acid). So you can
demethylate using strong base but the aldehyde will certainly react.
A procedure for any interested parties: http://www.orgsyn.org/Content/pdfs/procedures/CV3P0745.pdf"
.....in that procedure they employ the sulfur dioxide gas, i wonder if that 's really needed , hence conc. Hcl would be enough to knock off the K
......since the note says, "5. The sulfur dioxide treatment prevents the formation of a very dark-colored product when the reaction mixture is
acidified with a strong acid."........solo
Reference Information
Fussion of vanillin
U. S. pat. 2,547,920
-------------------------------------------------------------------------
Reaction of Vanillin and its Derived Compounds IV The Caustic Fusion of Vanillin
Irwin Pearl
J. Am. Chem. Soc.
68, 2180 (1946).
Summary
Caustic fusion of vanillin below 240-245˚C results in very high yields of vanillic acid free from protocatechuic acid. Fusion of vanillin above
240-245˚C yields protocatechuic acid free from vanillic acid. The critical demethylation temperature varies somewht with the alkali-vanillin ratio.
Reactions of vanillin with strong alkali solution at elevated temperatures does not yield either of the acids.
Attachment: Fusion of vanillin-pat2547920.pdf (143kB)
This file has been downloaded 801 times
Attachment: Reactions of Vanillin and its Derived Compounds. IV.1 The Caustic Fusion of Vanillin.pdf (269kB)
This file has been downloaded 997 times
[Edited on 17-3-2016 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Arggg. What I was suggesting was....Vanillin to Piperonal, might be difficult. But....
Eugenol to Iso-Safrole, could be more do-able....Via de-methylation of Eugenol.....followed by Methylenation.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
.....but how to convert the protocatechuic acid to the desired protocatechuic aldehyde so the OH are not touched or changed.....however there is the
conversion of the acid to an acid chloride, then an ester reduced to the desired aldehyde......solo
[Edited on 18-3-2016 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Korialstrasz69, aldehyde is the first to react with free radicals. Also, the ring is not resistant to halogenation, as you might know
by 5-bromovaniliin procedure. Free radical reaction is not the way Williamson ether synthesis is done.
chemplayer.., I'm sure either you did not try AlCl3+pyridine or you did it wrong. It definitely works giving high yields. Already
mentioned AlBr3/ArNO2 is also 100% working, but requires the toxic solvent. AlBr3 and AlI3 can be prepared in a cold solvent (dichloromethane is known
to work, but it reacts with aluminium halides, leading to AlCl3 in the end).
I was able find your pyr+hcl and alcl+thiourea videos, I tried AlCl3-thiourea route long time ago and I did not manage to extract any decent amount of
protocatechualdehyde(PA) in the end. It's really hard to say anything about products without chromatography, and sadly I was not used to it those
days, and I haven't repeat the experiment.
FeCl3 can be used for detecting vanillin/PA: purple for vanillin, green for PA. NaNO2, then heating, then NaOH is another simple one, however, the
colors do not depend on the structure of the compound and are rather random (but still the same for the same compound). For PA colors are NaNO2 -
yellow-orange, heating - yellow-red, NaOH - yellow-red (same).
Can it be that your vanillin is not a 100% vanillin? Does it have a correct melting point?
zed, do you think isosafrole is more usefull than piperonal? Also, you have much less choice of reagents/conditions with eugenol -
you can't use no acidic reagents until you get safrole/isosafrole. Does the eugenol-PTC-AlI3 route even work? I'm pretty sure nobody have ever
suceeded doing it.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
What about protecting the carbonyl group first by forming its thioacetal? I recall the thioacetal being more resistant to basic and acidic solutions,
which a strong acid is needed for the ether cleavage.
The protocatechuic acid can be turned into the alkylketone using lithium hydride followed by alkyllithium, as seen in the cyclohexyl ketone
preparation on OrgSyn (http://www.orgsyn.org/demo.aspx?prep=CV5P0775). This would be a pain though because of the strict requirement of air and moisture exclusion, not
something I am equipped for at this time. The reduction of the -OH to -H sounds like an LAH or catalytic H reduction, but something would likely have
to be done with the hydroxy groups previously regarding the alkylation and reduction reactions.
If I were to do this, my first attempt would be to follow the procedure outlined in this paper (http://www.ncbi.nlm.nih.gov/pubmed/23127660) where vanillin is taken all the way to MDMA, and the demethylation is accomplished with pyridine and
AlCl3.
(clarification: follow the demethylation procedure in the paper, not take it all the way to MDMA. that would conflict with my desire to not be
incarcerated.)
[Edited on 18-3-2016 by Loptr]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
It seems that link doesn't provide a download of the PDF.
Attachment: Synthesis and impurity profiling of MDMA prepared from commonly available.pdf (1MB)
This file has been downloaded 1777 times
Here is another paper that mentions dealkylation of the ether using sulfuric acid, and the already mentioned lewis acid method.
[Edited on 18-3-2016 by Loptr]
Attachment: Bjorsvik.Liguori.Minisci.Synthesis.Vanillin.Isovanillin.Heliotropin.pdf (183kB)
This file has been downloaded 983 times
[Edited on 19-3-2016 by Loptr]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Does anyone have more info on the sulfuric acid demethylation?
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
byko3y - We've tried this many times now and with some variation in conditions and reactants, and we always get the same result which is ~10% yield.
Our vanillin is Rhodia food grade and then dessicated for a week to ensure it's water-free. The DCM is reagent AR grade and we've even tried drying it
with P2O5 to be sure.
The only thing we can think of is that the process we use to make the AlCl3 (zinc chloride and Al powder) is resulting in some zinc chloride impurity
sublimed into the AlCl3 product. The AlCl3 thus formed works brilliantly for Friedel-Crafts reactions, but perhaps the zinc interferes in this
particular case.
For example, we once tried reacting vanillyl alcohol with Lucas' reagent (ZnCl2 in HCl) to see if this would form the chloride - but the result was a
dark blue complex. Clearly some vanillin-like compounds and zinc are reactive together.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
ChemPlayer, have you attempted using magnesium iodide for demethylation?
Selective demethylation and debenzylation of aryl ethers by magnesium iodide under solvent-free conditions and its application to the total
synthesis of natural products
DOI: 10.1039/b916969e
Kai Bao,a,b Aixue Fan,a Yi Dai,b Liang Zhang,a Weige Zhang,*a Maosheng Chenga and Xinsheng Yao*b
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Interesting. Thanks for the reference.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Selective demethylation and debenzylation of aryl ethers by magnesium iodide under solvent-free conditions and its application to the total
synthesis of natural products
Kai Bao,a,b Aixue Fan
Organic & Biomolecular Chemistry
2009,Issue 24,pp. 5084-5090
DOI: 10.1039/b916969e
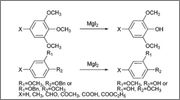
Abstract
An efficient selective demethylation and debenzylation method for aryl methyl/benzyl ethers using magnesium iodide under solvent-free conditions has
been developed and applied to the synthesis of natural flavone and biphenyl glycosides. A variety of functional groups including glycoside were
tolerated under the reaction conditions. Experimental results indicated that the removal of an O-benzyl group was easier than that of an O-methyl
group, regardless of wherever they were meta or para to the carbonyl. Thus selective debenzylation can be achieved for substrates bearing both
benzyloxy and methoxy groups.
Attachment: Selective demethylation and debenzylation of aryl ethers by magnesium iodide under solvent-free conditions and its appli (145kB)
This file has been downloaded 836 times
[Edited on 6-5-2016 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Loptr  | ChemPlayer, have you attempted using magnesium iodide for demethylation?
Selective demethylation and debenzylation of aryl ethers by magnesium iodide under solvent-free conditions and its application to the total
synthesis of natural products
DOI: 10.1039/b916969e
Kai Bao,a,b Aixue Fan,a Yi Dai,b Liang Zhang,a Weige Zhang,*a Maosheng Chenga and Xinsheng Yao*b |
Unfortunately it does not look like this method will demethylate vanillin. They make vanillin in two different ways (p-demethylation and
p-debenzylation) but with the p-hydroxy the m-methoxy seems protected (the do demethylate m-anisaldehyde).
[Edited on 7-5-2016 by careysub]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by careysub  | Quote: Originally posted by Loptr  | ChemPlayer, have you attempted using magnesium iodide for demethylation?
Selective demethylation and debenzylation of aryl ethers by magnesium iodide under solvent-free conditions and its application to the total
synthesis of natural products
DOI: 10.1039/b916969e
Kai Bao,a,b Aixue Fan,a Yi Dai,b Liang Zhang,a Weige Zhang,*a Maosheng Chenga and Xinsheng Yao*b |
Unfortunately it does not look like this method will demethylate vanillin. They make vanillin in two different ways (o-demethylation and
o-debenzylation) but with the o-hydroxy the m-methoxy seems protected (the do demethylate m-anisaldehyde). |
I did notice this but I hadn't evestigated it myself.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
There's not really good reason to bother with vanillin and eugenol when catechol is so much easier. If you're not trying to make drugs, benzodioxole
is about as useful as piperonal -- and if you are, it still is, but the routes are less well-known.
Interestingly, oxidizing salicylic acid with Fenton's reagent (Fe2+/H2O2) gives catechol-3-carboxylate.
Benzodioxole is most easily functionalized by chloromethylation:
https://www.erowid.org/archive/rhodium/chemistry/piperonal.n...
[Edited on 7-5-2016 by clearly_not_atara]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Just out of curiousity, what if you protected that p-hydroxyl group with O-alkylation? Would this be a non-issue then? I have read how notoriously
difficult this demethylation is, but what if both hydroxyl group were ethers.
|
|
|
| Pages:
1
2 |