| Pages:
1
2 |
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
Potassium Chlorate cell issue
I'm fairly new to chemistry in general but lately I've gotten into pyrotechnics and chemistry. I made some potassium chlorate from bleach for a while
but decided to order some titanium and mmo for an electrolysis cell. This is a 1 gallon Sterilite brand tub I've modified. I know it's not the perfect
setup or material but I want to see how much I enjoy chemistry in general before I order good lab equipment. My power supply is the typical computer
power supply with 3,5,12v, and ground easily accessible with RCA jacks. The vent tube is 1/4" vinyl and barb is nylon vented outside.
Anyway, I have a 40lb bag of potassium chloride and I ground 1200 grams of it in a blender and mixed it with 3 liters of distilled room temp water.
After 3 days of the cell running I have nothing more than a very fine layer of crystals on the bottom. From the research I've done I wasn't expecting
this little yield with such a big cell. It seems to be running fine and the cell is pretty hot to the touch (you can grab it for about 4 seconds).
What am I missing here or doing wrong?
 
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Which of the voltages are you running and how much current is it pulling? You need 60-70C temperature to get good chlorate production, otherwise
you're just making bleach or chlorine gas.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
i let mine run for weeks with the same stuff you got,i just kept refilling and adding chloride at 5 volts.i have made pounds and pounds of the
stuff.patience!
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
I was hoping patience would be one answer. I was pretty confident I had done enough research to get things going. You say you let it run for weeks...I
have no problem with that and I'm not in a hurry. I've just been a little frustrated after it running for 72+ hours and don't see anything
precipitating out. About how long would you say before you start getting consistent results?
From right to left, top to bottom or however the picture shows up when you click on it, the jack with the black wire is the ground, from there it goes
12v 5v 3v. I do have it one 5v jack. I'm not sure of the current as I didn't setup the power supply to be able to read it from a multimeter.
I finally found my thermometer and it's running exactly 60C.
[Edited on 22-2-2016 by Blake0577]
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
You get no precipitate untill the solution becomes saturated with chlorate. Apparantely you have just reached this point and you should get a faster
rate of product precipitating from here
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | I finally found my thermometer and it's running exactly 60C. |
Chlorate production will be much improved @ >80°!
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
If 60C/140F will work I will stick with that. That's about as comfortable as I can get with using Tupperware and holding those kind of temps for days.
Maybe it will hold more but it's definitely more soft/more flexible already.
One part I for sure failed on was using hot glue on the electrodes instead of plastic 2 part epoxy like I did the nylon barb. I'm not sure what I was
thinking on that one. How big of a deal would it be to stop it and clean up all the hot glue mess and apply epoxy?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | If 60C/140F will work I will stick with that. That's about as comfortable as I can get with using Tupperware and holding those kind of temps for days.
|
Use glass ─ a tall beaker or simple jar?
And cheap glass will do since resistive heating is uniform and unlikely to cause thermal stress . . . ?
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
I have a few other containers in mind for next time. I'm going to continue the run with what I have so hopefully it will hold out.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Hypochlorite disproportionates most readily above ~76° so that an increase of just ~16° will markedly increase yield . . .
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
I will try to bump the temp up to get there. Just thought about this...I dissolved the chloride in room temp water. So after the cell gets to
operating temp the solution is not saturated. Could this be part of the time delay?
By the way, please forgive the newbie questions. This is a great forum with loads of info. However, with something like a chlorate cell with so many
options, variables, and ways of achieving the same thing I knew I would end up with a few questions.
|
|
|
CrazyC
Harmless

Posts: 2
Registered: 28-12-2015
Member Is Offline
Mood: No Mood
|
|
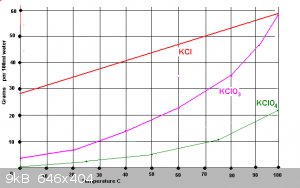
Here is a chart I got from the internet. It helped me understanding what going on and how much KCl03 water can hold in solution before precipitating
out. Good to know for recrytalization too.
Having one salt in solution is easy enofe to figure out so many gram per 100ml for a given temp. But in a your cell there are at lest two I'm not sure
how to figure that out I would like to know. I'll look into it when I get time.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Set a beaker down in your tank with some undissolved KCl. If it keeps disappearing, keep adding it back. This will also save an anode from low Cl
conditions.
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
I had to shut the cell down tonight. We have some bad storms coming in and I didn't want to risk having the electrodes sitting in the tank if the
power goes out. Plus the cell maintenance of trying to get the electrodes to seal and cleaning all the crust was getting old quickly. Before I shut it
down I checked the temp and it made it up to 85C after I added some insulation last night. Pretty impressive for the regular Tupperware container to
hold out like that although I will definitely be using acrylic next time. The biggest issue I would say is the typical leaking up the electrodes.
Thank you for all the responses. Any specific container recommendations with links would be appreciated.
[Edited on 24-2-2016 by Blake0577]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
While it still exists. Ignore the moving to URL, if it did its gone now.
http://www.oocities.org/capecanaveral/campus/5361/chlorate/c...
http://webpages.charter.net/dawill/tmoranwms/Chem_Chlorate.h...
http://www.pyrosecrets.com/chloratecell.html
http://www.vk2zay.net/article/86
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
I left my jug sitting outside last night and this is the yield after sitting out all day at 48F. Obviously not pure but not too bad for a first run
with electrolysis. I'm going to put it in a cooler with ice water overnight and see how much more I can get.
I really like the bucket cell adapter from Swede. I was a machinist for 10 years so that's right up my alley. I actually think I can make a few tweaks
and have a slightly better design.

|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
what made me impatient was making sodium chlorate because it took even more weeks.i would always interrupt to scoop out some chlorate to burn but it
stayed chloride for a long time.eventually i just ignored it and would just refill with water as it got low and one day it was all chlorate.my mmo
mesh electrodes from lasered were never harmed in any way and just kept producing.i used a medium sized plastic bucket(about 10" tall) at first i used
tupperware like plastic.the closer i would put the electrodes to each other,the hotter they became so would space them further apart.i found that
either i could get tougher material or lower amperage so i just spaced them further apart and that dropped the amperage(heat).on one run of potassium
chlorate i let it run forever to see how long the electrodes would last,i started getting a creamy like chlorate.the creamy chlorate burned twice as
fast.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by CrazyC  | Here is a chart I got from the internet. It helped me understanding what going on and how much KCl03 water can hold in solution before precipitating
out. Good to know for recrytalization too.
Having one salt in solution is easy enofe to figure out so many gram per 100ml for a given temp. But in a your cell there are at lest two I'm not sure
how to figure that out I would like to know. I'll look into it when I get time.
|
Due to common ionic effect the more soluble KCl (higher solubility constant equilibrium) will drop the solubility of KClO3 and KClO4 down...
K(+) + Cl(-) <--> KCl(s) (Ks1)
K(+) + ClO3(-) <--> KClO3(s) (Ks2)
K(+) + ClO4(-) <--> KClO4(s) (Ks3)
Ks1> Ks2 > Ks3
Ks1 = [K(+)] * [Cl(-)]
Ks2 = [K(+)] * [ClO3(-)]
Ks3 = [K(+)] * [ClO4(-)]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cyanureeves  | | on one run of potassium chlorate i let it run forever to see how long the electrodes would last,i started getting a creamy like chlorate.the creamy
chlorate burned twice as fast. |
Creamy like chlorate?!
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
it was all converted chlorate and it started to change into a non crystal substance that was deposited over the chlorate.it also was around the
electrodes also.when i scooped it out and mashed it with my hands it felt gritty like chlorate but the crystals were so tiny and compact.
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
Well after some crude filtering I ended up with 275 grams and lots of lessons learned! Realistically probably around 300+ but I wasn't too concerned
with maximum yield on this run. Still some chlorate left in the liquid I will reuse.

|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
that is completely normal
you will not get crystals until the solution is saturated
if your chloride has anti caking agents in it too i think it will take even longer
btw never use pure KCl as electrolyte because when it nears the end of the run your anodes will suffer as the chloride levels drop
that is only if they are MMO or Graphite and not platinum
add a filler like NaCl to prevent this so that all the KCl will be converted and it will start making NaClO3 at that point which means you can stop
since both NaCl and NaClO3 are very soluble then a simple washing removes them both
[Edited on 1-3-2016 by mysteriusbhoice]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Or if you don't wan the Na ions in your chlorate, you can just keep an open container of KCl in the reaction chamber to keep your Cl levels up.
|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
While I draft cad drawings of my version of the bucket cell adapter; I've made a new yet temporary cell. I found this on YouTube and it just so
happens I had been using this same container when I was doing the bleach method. It's only a 2 liter cell but it is acrylic and a much cleaner setup.
After 3 days or less it's already producing. I'm using KCl from Home Depot out of the 40lb bag. Farm grade I believe is the terminology used on this
forum. I used an old blender to grind 10lbs or so of the chunks into powder ready to use. I saturated a 2 liter solution at around 70C to mimic cell
conditions and quickly filtered it into the cell and cut the power on.
I also picked up a clamp meter to check amperage and it's running 4.4v at 19.5-20 amps. From what I've gathered that is about the max for the size MMO
anode I'm using. Temp is at 77C. I'm checking temp with a metal grilling/cooking thermometer (crude I know). Any suggestions for a lab/chemical grade
thermometer I could leave in the cell or for random checks?

|
|
|
Blake0577
Harmless

Posts: 15
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
Forgot to add: the white around the electrode seal is marine heat shrink tubing and epoxy, not leakage. If I can find a container that will hold up to
the conditions I will drop it in full of my powder. However, it was somewhere around 1,000g KCl to saturate the solution at 70c. No way possible it's
running low yet.
[Edited on 2-3-2016 by Blake0577]
|
|
|
| Pages:
1
2 |