khourygeo78
Harmless

Posts: 31
Registered: 30-12-2015
Member Is Offline
Mood: No Mood
|
|
Acetone - Acids reaction
Anyone has any idea about these reactions? and what do they yield? Or if there is anything readable on the web that can answer my question? I tried
searching but nothing unfortunately 
Thanks
Acetone + Sulfuric acid
Acetone + nitric acid
Acetone + hydrochloric acid
Acetone + citric acid
Acetone + acetic acid
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Look up the acid-catalyzed aldol condensation. I'm not sure off the top of my head if those organic acids are strong enough, but the mineral acids
certainly are. Sorry, I don't have time to explain further right now.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Sulphuric acid, I believe, will react with acetone to give trimethylbenzene.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
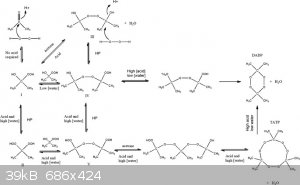
Acids do not "react" with ketones to produce new product to my knowledge. However, since acids have H+ ions, they are able to protonate the carbonyl,
effectively breaking the double bond as an intermediate. At this step, another compound is able to step in to fill the void made by the protonation.
The first example that comes to mind is the synthesis of TATP.
I am by no means knowledgable in organic; I'm simply a novice.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
D'you have a reference for that scheme?
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Of course!
Factors Influencing Triacetone Triperoxide (TATP) and Diacetone Diperoxide (DADP) Formation: Part 2
[edit] Idk why the hot link won't work when you click on it, you have to delete SciMad from the beginning
[Edited on 1-27-2016 by Detonationology]
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Acetone + Acid will yield in first a yellow-brown solution which will precipitate some black stuff and eventually if you wait long enough for the
Acetone to evaporate you will get a red-black oil. I tried that myself a few times to see what happens and it always ended in a polymer formed by
Aldol reactions.
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
What acid are you referring to?
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Conc H2SO4 is able to polymerise aceton into 1,3,5-trimethylbenzene (mesitylene).
Conc HCl will make lower polymer like phoron.
Conc HNO3 will lead you to a really vigorous runnaway (the all batch will boil at once in nxoy fumes with a short delay time depending on initial
temperature) and various undefined nitro/nitroso/oxydation resulting compounds.
Citric acid will yield nothing but the mix, just like acetic acid...
[Edited on 27-1-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Detonationology, all of the reaction mechanisms in that diagram you posted are only possible if hydrogen peroxide is present.
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Quote: Originally posted by Amos  | | Detonationology, all of the reaction mechanisms in that diagram you posted are only possible if hydrogen peroxide is present. |
Hmm... is the carbonyl not protonated by the acid regardless?
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@detonationology:
Please be more careful when citing, in the future. You pulled that graphic seriously out of context, as Amos showed. 
At LEAST browse the sources you are quoting.
[Edited on 28-1-2016 by blogfast25]
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
How so? Please explain in detail.
I can accept being incorrect about my original statement, just tell me why it is wrong or irrelevant.
We stand at the "Contradiction" level of "the pyramid of debate: "states the opposing case with little or no supporting evidence."
[Edited on 1-28-2016 by Detonationology]
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Eh, I really don't see hydrogen peroxide being "required" for Detonationology's point. That was the same basic mechanism as acid-catalyzed aldol
condensation or hemiacetal formation, just a different nucleophile.
That reaction diagram might have had different reactants, but it still shows how acids usually tend to protonate the carbonyl to facilitate the attack
of a nucleophile rather than incorporating into the final product. In this case we're looking at self-condensation where the enol form of acetone is
the nucleophile.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Etaoin Shrdlu  | Eh, I really don't see hydrogen peroxide being "required" for Detonationology's point. That was the same basic mechanism as acid-catalyzed aldol
condensation or hemiacetal formation, just a different nucleophile.
That reaction diagram might have had different reactants, but it still shows how acids usually tend to protonate the carbonyl to facilitate the attack
of a nucleophile rather than incorporating into the final product. In this case we're looking at self-condensation where the enol form of acetone is
the nucleophile. |
Maybe you should actually read it? W/o hydrogen peroxide NONE of the listed structures is possible. Hardly relevant to the OP's question though...
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Etaoin Shrdlu  | Eh, I really don't see hydrogen peroxide being "required" for Detonationology's point. That was the same basic mechanism as acid-catalyzed aldol
condensation or hemiacetal formation, just a different nucleophile.
That reaction diagram might have had different reactants, but it still shows how acids usually tend to protonate the carbonyl to facilitate the attack
of a nucleophile rather than incorporating into the final product. In this case we're looking at self-condensation where the enol form of acetone is
the nucleophile. |
Maybe you should actually read it? W/o hydrogen peroxide NONE of the listed structures is possible. Hardly relevant to the OP's question though...
|
"hardly relevant." Would you remind me what DOES occur when, say HCl, is combined with acetone? Please explain in great detail...
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Etaoin Shrdlu  | Eh, I really don't see hydrogen peroxide being "required" for Detonationology's point. That was the same basic mechanism as acid-catalyzed aldol
condensation or hemiacetal formation, just a different nucleophile.
That reaction diagram might have had different reactants, but it still shows how acids usually tend to protonate the carbonyl to facilitate the attack
of a nucleophile rather than incorporating into the final product. In this case we're looking at self-condensation where the enol form of acetone is
the nucleophile. |
Maybe you should actually read it? W/o hydrogen peroxide NONE of the listed structures is possible. Hardly relevant to the OP's question though...
|
Maybe you should actually read his post.
EDIT: Detonationology only ever proposed this as an example of the mechanism. "However, since acids have H+ ions, they are able to protonate the
carbonyl, effectively breaking the double bond as an intermediate. At this step, another compound is able to step in to fill the void made by the
protonation."
It was a perfectly good example of the mechanism. Stop grousing that the nucleophile is different.
[Edited on 1-28-2016 by Etaoin Shrdlu]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Grousing??? LOL
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Lol. That sure is one humble way of admit to being incorrect.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
khourygeo78
Harmless

Posts: 31
Registered: 30-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fluorescence  | | Acetone + Acid will yield in first a yellow-brown solution which will precipitate some black stuff and eventually if you wait long enough for the
Acetone to evaporate you will get a red-black oil. I tried that myself a few times to see what happens and it always ended in a polymer formed by
Aldol reactions. |
Interesting! Do you remember the smell of the red-black oil?
And didnt the acetone react with the acid to make a volatile compound (non oily). This can be perceived by smell&speed of evaporation.
|
|
|
khourygeo78
Harmless

Posts: 31
Registered: 30-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | Conc H2SO4 is able to polymerise aceton into 1,3,5-trimethylbenzene (mesitylene).
Conc HCl will make lower polymer like phoron.
Conc HNO3 will lead you to a really vigorous runnaway (the all batch will boil at once in nxoy fumes with a short delay time depending on initial
temperature) and various undefined nitro/nitroso/oxydation resulting compounds.
Citric acid will yield nothing but the mix, just like acetic acid...
[Edited on 27-1-2016 by PHILOU Zrealone] |
thanks. i just didnt understand what you meant by
"Conc HCl will make lower polymer like phoron". What does phoron mean here
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Phoron's just the name of a polymer.
Does anyone have a source for:
| Quote: | | Conc H2SO4 is able to polymerise aceton into 1,3,5-trimethylbenzene (mesitylene). |
Of course it seems that other users weren't able to perform this anyways.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by The Volatile Chemist  |
Does anyone have a source for:
| Quote: | | Conc H2SO4 is able to polymerise aceton into 1,3,5-trimethylbenzene (mesitylene). |
Of course it seems that other users weren't able to perform this anyways. |
I know which book I remember reading it in, but don't have it handy.
But it's here: http://www.orgsyn.org/demo.aspx?prep=CV1P0341
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Sorry, I probably should've checked OrgSyn.
That preparation of course differs on a few points from what has been described here. The tedious chilling, the steam-heating, etc.
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Thats a very OTC route to mesitylene. The yield at 13 to 15% is low but the reactants are cheap. I wounder if there is an other procedure that has a
better yield.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|