Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
P-Nitrophenol
I am going to perform the synthesis of P-Nitrophenol using a procedure largely based on the synthesis of P-Nitroaniline. I am wondering whether or not
the following reaction scheme would work:
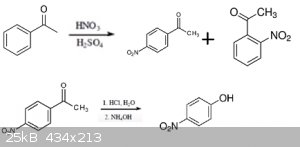
First is the nitration of the acetophenone which yields P and O nitroacetophenone. After isolation of the Ortho-product comes the acid hydrolysis of
the nitroacetophenone,the acid is then neutralized with ammonium or sodium hydroxide.
[Edited on 30-12-2015 by Agari]
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
Hi. You usually need peroxides or other strong oxidizers to cleave benzaldehydes and acetophenones to the phenols.
Where did you get this idea from?
Bromine, definitely bromine.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by NexusDNA  | Hi. You usually need peroxides or other strong oxidizers to cleave benzaldehydes and acetophenones to the phenols.
Where did you get this idea from? |
I got the idea from the reaction scheme for the synthesis of P-nitroaniline from acetanilide
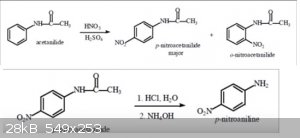
[Edited on 31-12-2015 by Agari]
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
That's the hydrolysis of an N-acetylamine, wildly different than the cleavage of a ketone. I would recommend preparing p-nitroaniline, then
diazotizing that to form the phenol (see Org Syn for details)
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
Oh ok. In the case of acetanilide it works because its the hydrolysis of an amide. You cant "hydrolyse" an acetophenone with just that, it would have
very high energy carbocation intermediates (wont happen). Search about this.
What you want for your synthesis is oxidative cleavage.
Bromine, definitely bromine.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by gdflp  | | That's the hydrolysis of an N-acetylamine, wildly different than the cleavage of a ketone. I would recommend preparing p-nitroaniline, then
diazotizing that to form the phenol (see Org Syn for details) |
Can you please provide a link to the specific article of interest?
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Here's an example for a different regioisomer.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Agari  |
First is the nitration of the acetophenone which yields P and O nitroacetophenone. After isolation of the Ortho-product comes the acid hydrolysis of
the nitroacetophenone,the acid is then neutralized with ammonium or sodium hydroxide.
|
you won't get significant ortho/para isomers if you nitrate acetophenone,you will get majority as meta
why don't you make p-nitrophenol from phenol itself ? there are many methods on SM using nitrate salts,catalysts (clay),and the good old
HNO2 followed by dilute HNO3 route .
[Edited on 31-12-2015 by CuReUS]
|
|
|