sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
could make formaldehyde that way?
could make formaldehyde that way?
I would place wood within an iron cylinder with two outputs, one for placing the timber and another for leaving the iron in the midst of iron put iron
oxide, immediately after the pipe would be frozen within a bowl with ice, leaving the methanal at the end
it works?
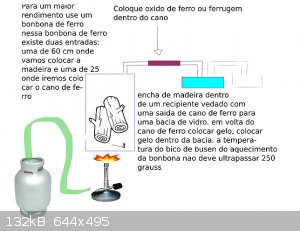
as I do not know anything about chemistry, my humble opnion would be:
ethanol would come along with various impurities, but I imagine that when passing through the iron oxide turns metanal being propio iron a catalyst?
based on what I saw on wikipedia:
Formaldehyde is produced industrially by the catalytic oxidation of methanol. The most common catalysts are silver metal or a mixture of an iron and
molybdenum or vanadium oxides. In the commonly used formox process, methanol and oxygen react at ca. 250-400 ° C in presence of iron oxide in
combination with molybdenum and / or vanadium to produce formaldehyde According to the chemical equation: [7]
2 CH3OH + 2 CH2O + O2 → 2 H2O
The catalyst silver-based Usually operates at a higher temperature, about 650 ° C. Two chemical reactions on it simultaneously produce formaldehyde:
that shown above and the dehydrogenation reaction:
CH 3 OH + H 2 → H2CO
In principle, formaldehyde Could be generated by oxidation of methane, but this route is not industrially viable because the methanol is more easily
oxidized than methane.
sorry for the dumb question'm still an apprentice
[Edited on 16-12-2015 by sclarenonz]
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Not realy a workable idea you would need to separate the methanol from the outher products of the destructive distillation first you need to use as
pure .methanol as you can get tto stop your catalyst from bein poisoned .
Aquarium shops sell formeldyhyde solution check around or but the methanol at a rc hobby shop.
You can make your own formeldyhyde but the best concentration ive got in one run through the catalyst is 30% you have to vacuum concentrate it to get
rid of excess methanol and to bring the % up to 37%.
|
|
|
ariep641514
Harmless

Posts: 6
Registered: 29-12-2015
Member Is Offline
Mood: No Mood
|
|
you can use copper tubing for AC, put positive pump to pump methanol + oxygen gas and give check valve input and output to maintain pressure, coil
coppertube heated with induction heater and dont forget to recooling before outlet. it will formed mixed methanol and formaldehyde solution. more easy
to get and simple design, smaller tube and more length will increase yield.
methanol more easy to get. as long u know where to find it 
[Edited on 30-12-2015 by ariep641514]
|
|
|
Texium
|
Thread Moved
29-12-2015 at 20:43 |