morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
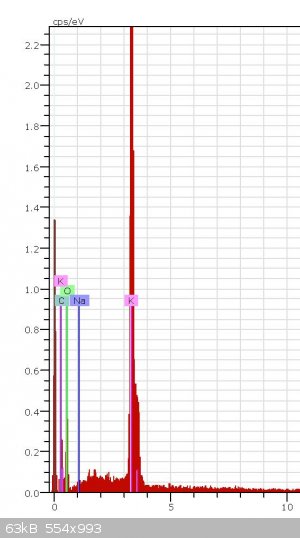
XPS identification
Can somebody know from this XPS graph what it is (-COOK or K2CO3) and how did you know it?
Thank you very much
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
This does not look like an XPS spectra. This looks more like EDS. The software that plots this data is capable of giving you estimates of atomic
percentages for each element present, and the percentages can give you an idea of what the compound could be, but it could also just be a mixture of
several compounds containing these elements. It would be pretty difficult to say which compound this spectra belongs too because EDS tells us nothing
about the bonding between the elements. On the other hand XPS does discern the bonding energies, therfore if we had high resolution XPS scans of the
oxygen, potassium and carbon peaks from an XPS spectra then perhaps we would be able to say which compound it was.
So, from this spectra all you can say is that these elements are present in your sample and if you normalize the peak intensities and take their
ratios you may be able to guess a compound, but it would not be conclusive.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
This seems like a lot of trouble to go to in order to answer a question that could be addressed by seeing if the stuff fizzes when you add vinegar.
|
|
|
morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
And how "deep" can EDS go? If I will have 20μm thick layer of for example zinc on iron will there be peak of iron on scan?
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Depends on density and accelerating voltage
 
pg. 135,
ISBN: 1-56677-041-6
[Edited on 10-4-2015 by WGTR]
|
|
|