bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
Any cool reactions with the use of Arsenic?
Arsenic or arsenates in general. A while back I got some arsenic acid off of the element by putting in nitric acid and thats about it. I have a bit
more than what I need to save for a sample of the element so if you guys know of or can come up with something cool then I'd like to hear it.
Thanks
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What kind of reaction would be considered 'cool' ?
|
|
|
bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
something thats unique or produces a compound with unique properties that could be observed
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Perhaps, if you're interested in compounds of historic importance, you could prepare Paris green?
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
From Experimenting with Chemistry: Experiments for the Home Lab by Burton Hawk:
 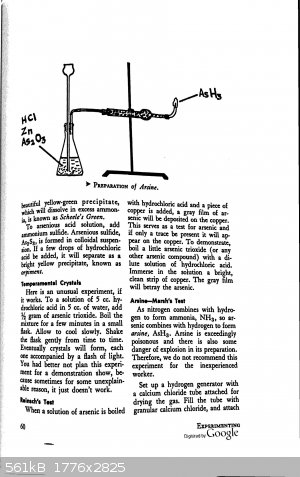 
Have fun and please be careful!
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
"In general, we do not recommend arsenic for suicide as it is slow, agonizing, and usually fatal."
Isn't that... kind of the point? The fatal part, I mean. :/
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Morkva
Harmless

Posts: 17
Registered: 6-11-2014
Member Is Offline
Mood: No Mood
|
|
Dude... cacodyl...
http://www.carrotmuseum.co.uk/falcarinol.html
“Science is the ultimate pornography, analytic activity whose main aim is to isolate objects or events from their contexts in time and space. This
obsession with the specific activity of quantified functions is what science shares with pornography.”
- J.G. Ballard, The Atrocity Exhibition
|
|
|
bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
the main problem is getting the arsenic trioxide.... I tried by burning the metal but i only got a very very thin layer of the oxide and the rest was
just gone...
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
From Wikipedia~
| Quote: |
"Cacodyl, dicacodyl, tetramethyldiarsine, alkarsine or minor part of the "Cadet's fuming liquid" (after the French chemist Louis Claude Cadet de
Gassicourt) (CH3)2As—As(CH3)2 is a poisonous oily liquid with a garlicky odor. Cacodyl undergoes spontaneous combustion in dry air."
|
Defiantly sounds interesting, perhaps not too amateur friendly though. Be safe.
Preperation, agian, from wikipedia.
| Quote: | Cacodyl oxide is prepared by the reaction of potassium acetate with arsenic trioxide.
4 CH3CO2K + As2O3 → As2(CH3)4 + 2 K2CO3+ 2 CO2
A subsequent reduction or disproportionationof the substance under the reaction conditions yields a mixture of several methylated arsenic compounds.
A far better synthesis was developed which started from the dimethyl arsine chloride and dimethyl arsine.
As(CH3)2Cl + As(CH3)2H → As2(CH3)4 + HCl |
[Edited on 23-8-2015 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Quote: Originally posted by bolbol  | | the main problem is getting the arsenic trioxide.... I tried by burning the metal but i only got a very very thin layer of the oxide and the rest was
just gone... |
Perhaps make arsenic trichloride from your elemental arsenic, and then hydrolysis to arsenic trioxide.
| Quote: |
Arsenic trichloride...
prepared by chlorination of arsenic at 80–85 °C, but this method requires elemental arsenic.
2 As + 3 Cl2 → 2 AsCl3
|
| Quote: |
Arsenic trioxide
In the laboratory, it is prepared by hydrolysis of arsenic trichloride
2 AsCl3 + 3 H2O → As2O3 + 6 HCl
|
[Edited on 23-8-2015 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Does anyone have this pdf? It was mentioned a few years ago on this site, with the statement that it was on Google Books neither of which seems true.
|
|
|
bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bot0nist  | Quote: Originally posted by bolbol  | | the main problem is getting the arsenic trioxide.... I tried by burning the metal but i only got a very very thin layer of the oxide and the rest was
just gone... |
Perhaps make arsenic trichloride from your elemental arsenic, and then hydrolysis to arsenic trioxide.
| Quote: |
Arsenic trichloride...
prepared by chlorination of arsenic at 80–85 °C, but this method requires elemental arsenic.
2 As + 3 Cl2 → 2 AsCl3
|
| Quote: |
Arsenic trioxide
In the laboratory, it is prepared by hydrolysis of arsenic trichloride
2 AsCl3 + 3 H2O → As2O3 + 6 HCl
|
[Edited on 23-8-2015 by Bot0nist] |
Yeah I've been thinking for a while on how I can make that work. I am still not sure if ill have to maintain the temperature at 80 degrees or it will
turn into a chain reaction and if it does I need to know how much heat will be released from it so I gotta do a small scale.
By the way wikipedia mentions that As2O3 goes through hydrolysis as well producing arsenous acid. It also mentions that arsenous acid only exists in
solution so hopefully evaporating the water will give me back the As2O3?
Any other ways for the making of As2O3? Or any other way to turn arsenic acid into arsenous acid and evaporate it for As2O3?
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
You could try synthesising this :- 
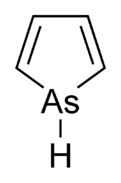
If you're not part of the solution, you're part of the precipitate.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
If you were able to make Arsenite from it. Sodium Arsenit is a secondary standard.
It's nothing dramatic but it's always good to have solutions where you know the
exact concentration for titration.
Otherwise there is quite a lot about arsenic chemistry in Gmelin. But that Pyroll with Arsenic
looks really cool. I'd love to make that one, too.
There is also quite a lot you can do with it
http://www.chm.bris.ac.uk/sillymolecules/arsole2.pdf
I dunno. I had Organic Chemistry III last semester which was one semester full or Heterocycles.
We looked at Stuff like Furane and Pyrrole and at it's reactivity and possible reactions but we
never had Arsole. Might be worth looking a bit deeper into that matter although I'm not really sure
what you'd need that molecule for.
Edit:
How would you synthezise it ? Pyrrole substitutes are usually made by
just closing the already substituted linear molecule to a ring. But pyrrole itself doesn't really work that way. You usually make it from Furane with
Ammonia. So perhaps with a good catalyst and AsH3 ?
[Edited on 23-8-2015 by fluorescence]
|
|
|
Tabun
Harmless

Posts: 38
Registered: 17-4-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | "In general, we do not recommend arsenic for suicide as it is slow, agonizing, and usually fatal."
Isn't that... kind of the point? The fatal part, I mean. :/
|
I think it depends on how you pronounce it.It can also sound like "not always",or at least to me and people like me.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Please post your intentions, and an Update at least once a day bobol so we can see how long whatever compound you make takes to kill
you.
If you die making an As compound, the least you can do is give us some information about how it smelt or tasted before you die.
|
|
|
bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
I have a thick skin to be honest but will do as soon as I start working on it. Ill taste some arsenic acid and let u know since I already have that.
And dont say "No, youll die!" lol
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I would not try to make volatile arsenic compounds. Steer away from AsCl3, AsH3. Burning of As also does not sound like something which I would like
to do in/near my house.
Aqueous experiments with As can be done though if done with care.
You could try dissolving some arsenic in HNO3 and then neutralizing the solution with a base and then adding the resulting solution to a strong
solution of a sulfide. This will give you thiosalts of arsenic (e.g. thioarsenate). When such salts are acidified, then you get H2S (be careful with
that as well) and a precipitate of arsenic sulfide(s), which can have a beautiful orange or yellow color.
[Edited on 24-8-15 by woelen]
|
|
|